Treatment of lymphoid neoplasms with CAR-T cells
Tratamento de neoplasias linfoides com células CAR-T
Andreza de J. Silva1, Patrick Menezes2, Diogo Felipe Corecha do Nascimento3
1 Universidade do Grande Rio “Prof. José de Souza Herdy” – UNIGRANRIO AFYA, Curso de Biomedicina. Duque de Caxias, RJ, Brazil.
2 Hospital Universitário Gaffrée e Guinle (HUGG-UNIRio/Ebserh), Unidade de Laboratório de Análises Clínicas e Anatomia Patológica. Preceptor Científico do Curso de Biomedicina. Rio de Janeiro, RJ, Brazil.
3 Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti (HEMORIO), Laboratório de Citologia. Rio de Janeiro, RJ, Brazil.
Received on 31/01/2024
Approved on 21/05/2024
DOI: 10.21877/2448-3877.202500164
INTRODUCTION
Lymphoid neoplasms arise from the malignant transformation of cells in the lymphoid tissue at various stages of their natural maturation process.(1) Among these neoplasms, we can highlight acute lymphoblastic leukemia (ALL), which originates from a lymphoid progenitor cell capable of differentiating into T or B lymphocytes; chronic lymphocytic leukemia (CLL), which arises from the transformation of a more mature B lymphocyte progenitor; and multiple myeloma, which involves a cell at an even later stage in B lymphocyte maturation.(2) The World Health Organization (WHO) recognizes a variety of lymphoid neoplasms, classified according to their immunophenotypic, genetic, and clinical characteristics.(3,4)
Neoplasms in this group exhibit not only a great morphological diversity but also a broad spectrum of clinical manifestations.(1) This diversity is reflected in the treatments to which these pathologies are responsive.(1) In cases of treatment failure or relapse after the initial treatment, stem cell transplantation is considered.(1) Refractory disease and relapse are two of the main challenges in the treatment of hematological neoplasms.(1) Several immunotherapy modalities have shown promise in attempting to induce long-term remission in refractory or relapsed neoplasms.(5,6)
Despite significant therapeutic advances in recent years, lymphoid neoplasms still present a poor prognosis, and therapeutic alternatives have been the focus of research and clinical trials.(5) In this context, chimeric antigen receptor T cell (CAR-T) therapy has shown promising results, with the FDA (U.S. Food and Drug Administration) approving five CAR-T cell-based treatments for hematological neoplasms.(5) This treatment relies on the reprogramming of the patient’s own cells and directing them against tumor cells.(7)
CAR-T cells are genetically modified immune system cells (Figure 1) designed to express a chimeric receptor specific to a surface antigen found on malignant cells.(8) These chimeric receptors are engineered to specifically target and recognize tumor cells, activating a more potent and targeted immune response against the malignant cells (Figure 2).(9,10) In the case of lymphoid neoplasms, CAR-T cells are directed towards specific antigens found on neoplastic lymphoid cells.(10) These target antigens can vary depending on the type of lymphoid neoplasm.(11) By targeting these antigens, CAR-T cells can selectively eliminate tumor cells (Figure 3),(12) sparing healthy cells of the lymphatic system.(13)
The trajectory of CAR-T therapy (Figure 4), from its origins to its spread in Brazil, is a narrative of innovation and hope in cancer treatment.(14) In this context, the objective of this review was to analyze studies related to CAR-T cells, lymphoblastic leukemia, and lymphoma over a five-year period. The research utilized the PubMed database as a source of information to observe the state of the art.
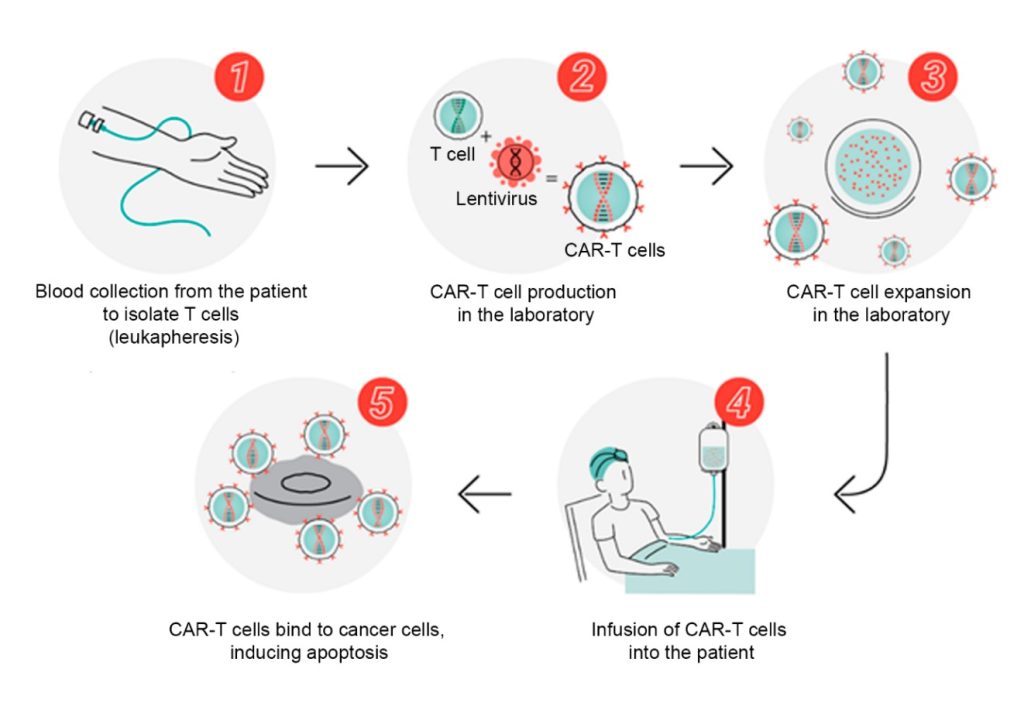 Figure 1
Figure 1
CAR-T cell production process.
Source: Adapted from Butantan; USP; Hemocentro de Ribeirão Preto, 2022.
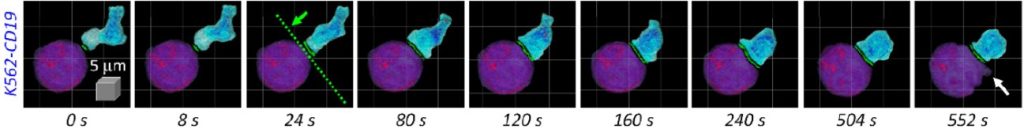
Figure 2
Mechanism of action of CAR-T cells.
Source: Lee et al., 2020.
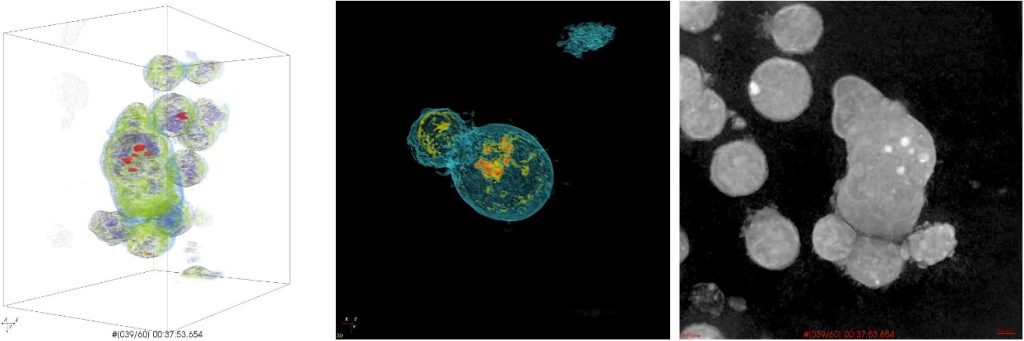 Figure 3
Figure 3
Visualization of holotomography, a microscopy technique that allows real-time, three-dimensional imaging of CAR-T cell behavior in response to target cells.
Source: Lee et al., 2019
| 1987 | Creation of the first chimeric antigen receptor (CAR)
In this year, scientists made a fundamental breakthrough by developing the first CAR, also known as the Chimeric Antigen Receptor. This CAR was incorporated into T cells, creating CAR-T cells. These modified cells began to express specific receptors, allowing them to identify and bind to tumors. This discovery sparked a revolution in cancer therapy. |
| 1992 | Use of retroviral vectors to introduce genes into T cells
American immunologist Michael Sadelain, from MIT, began using retroviral vectors to introduce genes into T cells. The goal was to modify these cells to target specific tumors. |
| 1994 | Isolation of specific T cells
Researchers at Memorial Sloan Kettering Cancer Center (MSK) in New York learned to isolate specific T cells for use in stem cell transplants, aiming to prevent virus-induced tumors. |
| 1998 | Introduction of the co-stimulatory molecule CD-28
Michael Sadelain’s team introduced the co-stimulatory molecule CD-28 into modified T cells (CAR-T), allowing them to remain active in the body and making them more effective in fighting cancer. |
| 2002 | Construction of the first effective CAR-T cells in vitro
Researchers at Memorial Sloan Kettering Cancer Center (MSK) constructed the first effective CAR-T cells, targeting specific cancer antigens. These cells demonstrated the ability to survive, multiply, and kill cancer cells in laboratory tests, validating the viability of the therapy. |
| 2003 | CAR-T cells kill leukemia cells in animals
The research group at MIT demonstrated that CAR-T cells targeting CD-19 were capable of killing leukemia cells in mice. |
| 2009 | Establishment of the CAR-T cell manufacturing process
The same MIT team pioneered the development of the CAR-T cell manufacturing process, engineering the cells to target CD-19 for the treatment of patients with chemotherapy-resistant and relapsed leukemia. The success of the process and the effectiveness of the cells were confirmed. |
| 2010 | First leukemia patients cured with CAR-T therapy
Two patients with terminal-stage chronic lymphocytic leukemia volunteered for the first clinical trial of CAR-T therapy, conducted at the University of Pennsylvania. Both achieved complete remission and remain cancer-free to this day. CAR-T cells are still detectable in their bodies a decade later. |
| 2012 | First child with leukemia receives CAR-T therapy
At the age of 7, Emily Whitehead became the first child to receive CAR-T therapy. She was hospitalized with terminal leukemia and was successfully treated by Stephan Grupp’s team at the Children’s Hospital of Philadelphia. The CAR-T cells saved her life, and she achieved complete remission. |
| 2017 | FDA approval of CAR-T
After several successful clinical trials, the U.S. Food and Drug Administration (FDA) approved CAR-T for the treatment of terminal cases of leukemia and lymphoma. Currently, the FDA has approved five different CAR-T therapies. |
| 2019 | First patients receive CAR-T in Brazil
The Cell Therapy Center at the Hemocentro de Ribeirão Preto (CTC-USP) administered experimental CAR-T therapy to patients with blood cancers, such as lymphoma and leukemia, who had no other treatment options. Most of these patients achieved remission. |
| 2022 | Creation of the Cell Therapy Program at the Butantan Institute, USP, and Hemocentro
CAR-T technology spread throughout Brazil with the establishment of new cell therapy production centers for cancer by the Butantan Institute, the Universidade de São Paulo (USP), and the Hemocentro de Ribeirão Preto. These units have the capacity to treat hundreds of patients annually, marking an important milestone in access to CAR-T therapy in the country. |
Figure 4
Key milestones in the journey of CAR-T cell therapy.
Source: Adapted from Moon, 2023.(14)
MATERIALS AND METHODS
The research was conducted exclusively using scientific texts for the literature review, which relied solely on academic-scientific bibliographic sources. This approach was based on the authors’ experience with the topic at a specialized onco-hematology center, which, according to the Resolution of the National Health Council (CNS) number 510 of 2016, is exempt from registration and evaluation by the CEP/Conep System. The objective was to analyze studies related to CAR-T cells, lymphoblastic leukemia, and lymphoma over a five-year period. The research utilized the PubMed database as a source of information to assess the state of the art.
For the selection of studies, inclusion criteria were established, which encompassed the availability of free full-text articles and the inclusion of clinical trials. Conversely, exclusion criteria included studies involving other types of neoplasms.
The aim of this research was to provide an updated overview of the use of CAR-T cells in the treatment of lymphoblastic leukemia and lymphoma, exploring the clinical trials available in the scientific literature over the last five years. The literature review enabled the compilation and analysis of relevant studies in the field, contributing to the understanding of advances and challenges in this area.
RESULTS
Ramos et al. (2018) detailed an extremely relevant clinical case involving a 52-year-old male patient diagnosed with diffuse large B-cell lymphoma.(10) The patient’s historical context provided important information about his treatment journey.(10) The patient’s clinical history is noteworthy due to his previous treatment.(10) Autologous stem cell transplantation is a standard procedure that involves the collection of the patient’s own stem cells, followed by high-dose chemotherapy, and ultimately the reinfusion of the stem cells to aid in the recovery of the hematopoietic system.(10) However, it is concerning to observe that despite this previous treatment, the patient experienced a relapse of the disease.(10) A relapse indicates that the lymphoma reappeared after an initial period of remission, suggesting that the previous treatment was insufficient to control the disease’s progression in the long term.(10) In this context, the clinical case description suggests that CAR-T cells were considered as a treatment option following the failure of the prior treatment.(10) This approach is noteworthy because CAR-T therapies have shown efficacy in treating certain types of lymphomas, especially when other treatments have been unsuccessful.(10)
The patient in question underwent a treatment that included CAR-T cell therapy following a lymphodepleting chemotherapy phase.(10) Lymphodepletion is an important step prior to CAR-T cell infusion, as it helps create a more favorable environment for these modified cells to act effectively, reducing competition with the patient’s immune system cells.(10) A crucial step in evaluating the response to treatment was the performance of a second PET scan (Figure 5), which took place 6 weeks after the CAR-T cell infusion.(10) This examination is essential for monitoring cancer spread and evaluating treatment efficacy.(10) The results of this second PET scan were highly encouraging, indicating a complete response (CR).(10) A complete response on a PET scan is a significant milestone in the treatment of cancer patients.(10) This means that, according to the images obtained, no areas were identified in the body showing abnormal metabolic activity, suggesting the near-complete elimination of cancer cells.(10) This is a remarkable achievement, indicating that the CAR-T cell treatment had an extremely positive impact on the control and regression of diffuse large B-cell lymphoma.(10)
Ramos et al. (2018) also present a clinical case involving a 67-year-old male patient diagnosed with diffuse large B-cell lymphoma.(10) The patient’s history is notable for his complex treatment journey.(10) After failing to proceed to stem cell transplantation despite undergoing two rescue regimens, the patient was subjected to a protocol involving lymphodepletion followed by CAR-T cell infusion.(10) However, the patient’s progress after CAR-T cell therapy was marked by notable complications.(10) Ten days after the CAR-T cell infusion, he developed fever and tachypnea, which led to his hospitalization.(10) The occurrence of these symptoms is concerning, as it may suggest an adverse reaction to the treatment or the presence of complications associated with CAR-T therapy.(10) Analyzing the patient’s inflammatory markers, including C-reactive protein (CRP), which peaked at 12.2 mg/dL on day 11, and interleukin 6, which increased from 6.3 pg/mL at the beginning of the study to a peak of 91.2 pg/mL on day 11, revealed an elevation.(10) These results indicate that the patient was experiencing mild cytokine release syndrome (CRS).(10)
Abdo et al. (2020) presented Kaplan-Meier graphs used to compare and analyze the outcomes of two patient groups treated with different therapeutic approaches related to CAR-T cells.(15) A notable aspect of this study was that the patients were divided into two distinct groups: one that received newly modified CAR-T cells using the point-of-care approach, and another that received CAR-T cells that were expanded for 8 days and cultured according to the traditional protocol.(15)
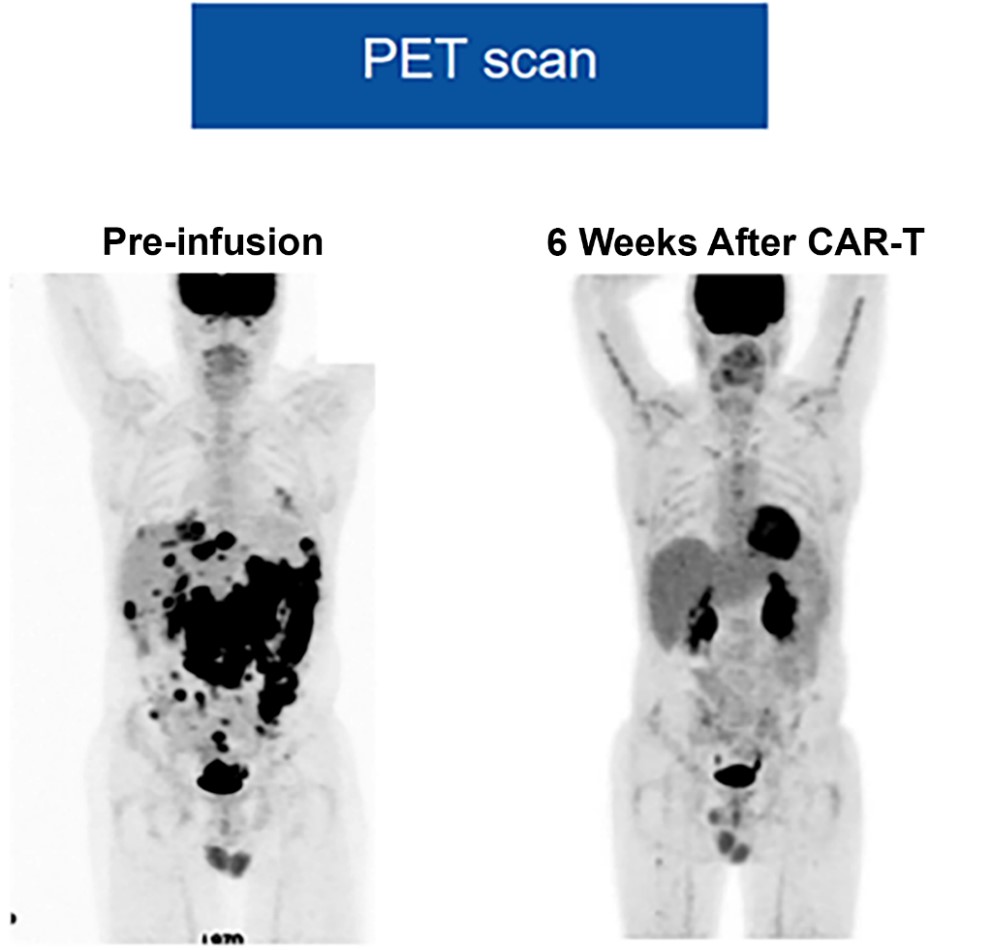
Figure 5
PET scan visualization before CAR-T infusion and 6 weeks post-infusion, showing a complete response.
Source: Adapted from Ramos et al., 2018.
DISCUSSION
The imaging study conducted 6 weeks after the infusion of CAR-T cells is an essential step in monitoring patients undergoing this treatment,(10) typically carried out through a PET scan or positron emission tomography.(10) The PET scan is a highly sensitive tool that allows the detection of areas in the body that remain metabolically active.(10) This metabolic activity may indicate the presence of residual cancerous cells, which is crucial for evaluating the effectiveness of CAR-T cell therapy.(10) The interpretation of the PET scan results is fundamental in categorizing the disease’s progression and determining the treatment response.(10) The established criteria—complete response, partial response, progressive disease, and stable disease—serve as an objective basis for assessing treatment efficacy.(10) A complete response indicates the total elimination of cancerous cells, while a partial response suggests a significant reduction in the tumor.(10) In contrast, progressive disease indicates that the patient’s condition is worsening, and stable disease suggests no significant changes in the disease.(10) The combination of advanced imaging technology, such as the PET scan, and well-defined response criteria allows for a precise and objective assessment of the effectiveness of CAR-T cell therapy.(10) This, in turn, aids in making important clinical decisions and adapting treatment to meet each patient’s specific needs, thereby improving outcomes.(10)
The combination of chemotherapy-induced lymphodepletion followed by CAR-T infusion, along with cytotoxicity assays using CRP and interleukin 6 (IL-6), represents a comprehensive and integrated approach for the treatment and evaluation of efficacy in patients undergoing CAR-T therapy.(16) The inclusion of these biological markers, CRP and IL-6, in clinical trials may be significant for several important reasons.(16) First, CRP is a protein produced in response to inflammation in the body.(16) When CRP levels increase after CAR-T infusion, this may indicate an inflammatory response to the destruction of cancer cells by CAR-T cells.(16) This information is valuable as it can help monitor and understand the patient’s immune responses to the treatment.(16) A sharp increase in CRP levels may suggest a robust immune response, which could be desirable in certain therapeutic contexts, while moderate or stable levels may indicate a more controlled response.(16) Secondly, interleukin 6 (IL-6) is a cytokine that plays a fundamental role in regulating the immune response and modulating inflammation.(16) Measuring IL-6 levels can provide important insights into how the immune system is being activated or modulated by CAR-T cells.(16) This is essential for understanding not only the treatment’s effectiveness but also the potential side effects and complications associated with CAR-T therapy.(16) Proper control of the inflammatory and immune response is crucial for the safety and well-being of patients.(16) Therefore, the inclusion of CRP and IL-6 in clinical trials related to CAR-T therapy provides a detailed view of the body’s response to the treatment.(16) These markers can help healthcare professionals adjust and personalize therapy according to each patient’s individual needs, maximizing therapeutic benefits and minimizing potential risks.(16) Furthermore, this integrated approach contributes to the advancement of scientific knowledge in the field and the continuous improvement of CAR-T therapy as an effective treatment option for cancer patients.(10)
A common approach in preclinical research to assess the efficacy and safety of CAR-T therapy involves the use of immunodeficient mouse models that have been previously engrafted with different types of B-cell leukemia.(15) The genetic modification process used in these studies is mediated by a transposon known as “Sleeping Beauty,” and a notable aspect is the application of the point-of-care technique, which refers to a rapid and localized process carried out in the laboratory itself, without the need to send samples or experiments to distant facilities.(15)
The PET scan examination is an essential tool in the pre-infusion stage for mapping cancer spread and identifying areas of tumor activity.(10) It is an exam that uses a radioactive substance called a radiopharmaceutical to visualize the body’s cells that consume more glucose, such as cancer cells.(10) Achieving a complete response on the PET scan after CAR-T cell treatment is a highly positive outcome and indicates the treatment’s efficacy.(10) When we refer to a complete response, we mean that there is no visible evidence of tumor activity in any part of the body that has been evaluated.(10) This means that all previous tumor lesions, which were detectable on the pre-treatment PET scan, are no longer present or show no metabolic activity, which is an encouraging sign that the treatment is succeeding.(10)
Comparing the results of a PET scan performed 6 weeks after CAR-T infusion with a previous study conducted 6 months post-infusion allowed us to evaluate two distinct phases of treatment and provide valuable insights into the patient’s progress.(17) The 6-week scan assesses the initial response and acute efficacy of the treatment.(17) At this point, it shows how the CAR-T cells are responding immediately to the cancer.(17) It is a critical window to identify any early signs of success or challenges in the treatment.(17) Conversely, the 6-month post-infusion study focuses on the stability of the complete response and long-term remission observation.(17) This is crucial for determining whether CAR-T therapy is maintaining its efficacy over time and whether the patient is experiencing a durable remission.(17) Both time points are essential for monitoring the patient’s progress after CAR-T therapy, providing a view from the immediate response to the long-term scenario.(17)
Therefore, in the PET scan conducted 6 weeks after the infusion of CAR-T cells, the initial efficacy is essential to assess the early response to treatment.(17) During the evaluation of the acute response, it is possible to observe the elimination of most target cells, leading to a significant reduction in tumor metabolic activity.(17) The initial complete response indicates that the treatment was effective, but it is important to emphasize that long-term follow-up is still necessary to ensure the durability of this response and to monitor for potential recurrences.(17)
However, 6 months after the infusion of CAR-T cells, evaluating the long-term response becomes crucial. This assessment aims to determine the stability of the complete response over time.(17) When remission consolidation is observed, it means that the initial response persists and is maintained, indicating a complete remission over time.(17) It is important to highlight that a favorable prognosis is associated with a sustained complete response.(17) This means that if the response persists, there is an optimistic outlook regarding the treatment outcome, with a significant reduction in the risk of relapse, providing greater hope and quality of life for the patient.(17)
Feng et al. (2020) observed that the intense activation of CAR-T cells after infusion can trigger the release of pro-inflammatory cytokines (Figure 6) into the bloodstream.(16) This, in turn, can lead to the occurrence of cytokine release syndrome, which typically manifests in the first days following CAR-T cell infusion.(16) This syndrome involves the rapid release of cytokines, such as interleukin 6, and can be monitored through levels of CRP, which plays a crucial role in evaluating the immune and inflammatory response.(16) Therefore, it is important to closely monitor these markers to manage the potential side effects of CAR-T cell therapy.(16)
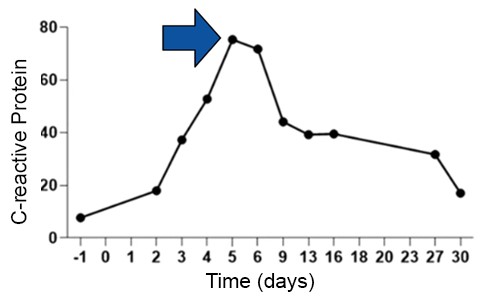
Figure 6
Measurement of C-reactive protein, showing mild cytokine release syndrome toxicity of grade I after CAR-T cell therapy.
Source: Adapted from Feng et al., 2020.
CONCLUSIONS
Based on the information and data presented, it is concluded that the studies and clinical trials conducted in this literature review demonstrate that CAR-T cell therapy has the potential to provide long-lasting remission in patients with lymphoid neoplasms, and that the management of side effects is a crucial aspect of the treatment.(18,19)
The assessment of response with PET scan before and after CAR-T cell infusion is essential for evaluating cancer spread and treatment efficacy.(20) A complete response on PET scan indicates an effective treatment and is a positive milestone in patient monitoring.(21) Comparing PET scan results performed at different time points after CAR-T cell infusion is crucial.(22) The PET scan performed 6 weeks after the infusion evaluates the initial response and acute efficacy of the treatment, while a PET scan performed 6 months after the infusion examines the stability of the complete response and long-term prognosis.(23,24)
Cytokine release syndrome is a potential side effect of CAR-T cell therapy, which is associated with elevated levels of pro-inflammatory cytokines, such as interleukin 6, and C-reactive protein.(25) Monitoring these markers is important for the identification and management of cytokine release syndrome.(26)
In summary, the studies presented highlight the importance of careful evaluation of the response to CAR-T cell therapy, the challenges associated with cytokine release syndrome, and the viability of point-of-care as a more accessible and effective approach.(27) These advancements have the potential to significantly improve the treatment of cancer patients, providing better outcomes and accessibility.(28)
REFERENCES
- Okikiolu J & McNamara C. (2015). Lymphoid neoplasms. Hematology (Amsterdam, Netherlands), 20(3), 182–183. Available at: https://doi.org/10.1179/1024533215z.000000000351
- Hanel W, Shindiapina P, Bond DA, Sawalha Y, Epperla N, Voorhees T, Welkie RL, et al. (2023). A phase 2 trial of ibrutinib and nivolumab in patients with relapsed or refractory classical Hodgkin’s lymphoma. Cancers, 15(5), 1437. https://doi.org/10.3390/cancers15051437
- Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, et al. (2007). Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood, 110(2), 695-708. Available at: https://doi.org/10.1182/blood-2006-11-051672
- Duffield AS, Mullighan CG & Borowitz MJ (2023). International Consensus Classification of acute lymphoblastic leukemia/lymphoma. Virchows Archiv: An International Journal of Pathology, 482(1), 11–26. Available at: https://doi.org/10.1007/s00428-022-03448-8
- Pasqui DM, Latorraca CdOC, Pacheco RL & Riera R (2022). CAR‐T cell therapy for patients with hematological malignancies. A systematic review. European Journal of Haematology, 109(6), 601-618. Available at: https://doi.org/10.1111/ejh.13851
- Tang L, Huang Z, Mei H & Hu Y (2023). Immunotherapy in hematologic malignancies: achievements, challenges and future prospects. Signal Transduction and Targeted Therapy, 8(1), 1-39. Available at: https://doi.org/10.1038/s41392-023-01521-5
- Haslauer T, Greil R, Zaborsky N & Geisberger R (2021). CAR T-cell therapy in hematological malignancies. International Journal of Molecular Sciences, 22(16), 8996. Available at: https://doi.org/10.3390/ijms22168996
- Butantan; USP; Hemocentro de Ribeirão Preto. Terapia contra o câncer com células CAR-T. Butantan, 2022. Available at: https://terapiacelular.butantan.gov.br/
- Lee M, Lee Y-H, Song J, Kim G, Jo Y, Min H, Kim CH & Park Y (2020). Deep-learning-based three-dimensional label-free tracking and analysis of immunological synapses of CAR-T cells. eLife, 9. Available at: https://doi.org/10.7554/elife.49023
- Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, Mehta B, et al. (2018). In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Molecular Therapy: The Journal of the American Society of Gene Therapy, 26(12), 2727-2737. Available at: https://doi.org/10.1016/j.ymthe.2018.09.009
- Gong W-J, Qiu Y, Li M-H, Chen L-Y, Li Y-Y, Yu J-Q, Kang L-Q, et al. (2022). Investigation of the risk factors to predict cytokine release syndrome in relapsed or refractory B-cell acute lymphoblastic leukemia patients receiving IL-6 knocking down anti-CD19 chimeric antigen receptor T-cell therapy. Frontiers in immunology, 13. Available at: https://doi.org/10.3389/fimmu.2022.922212
- Lee M, Lee Y-H, Song J, Kim G, Jo Y, Min H, Kim CH & Park Y (2019). Deep-learning based three-dimensional label-free tracking and analysis of immunological synapses of chimeric antigen receptor T cells. Em bioRxiv (p. 539858). Available at: https://doi.org/10.1101/539858
- Zhao L & Cao YJ (2019). Engineered T cell therapy for cancer in the clinic. Frontiers in Immunology, 10. Available at: https://doi.org/10.3389/fimmu.2019.02250
- Moon P. A história da terapia CAR-T: 60 anos de evolução e pioneirismo em direção à cura do câncer. Butantan, 2023. Available at: https://butantan.gov.br/noticias/a-historia-da-terapia-car-t-60-anos-de-evolucao-e-pioneirismo-em-direcao-a-cura-do-cancer
- de Macedo Abdo L, Barros LRC, Saldanha Viegas M, Vieira Codeço Marques L, de Sousa Ferreira P, Chicaybam L & Bonamino MH (2020). Development of CAR-T cell therapy for B-ALL using a point-of-care approach. Oncoimmunology, 9(1). Available at: https://doi.org/10.1080/2162402x.2020.1752592
- Feng J, Xu H, Cinquina A, Wu Z, Chen Q, Zhang P, Wang X, et al. (2021). Treatment of aggressive T cell lymphoblastic lymphoma/leukemia using anti-CD5 CAR T cells. Stem Cell Reviews and Reports, 17(2), 652-661. Available at: https://doi.org/10.1007/s12015-020-10092-9
- Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, Frank MJ, et al. (2021). CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nature Medicine, 27(8), 1419-1431. Available at: https://doi.org/10.1038/s41591-021-01436-0
- Tudor T, Binder ZA & O’Rourke DM (2021). CAR T cells. Neurosurgery Clinics of North America, 32(2), 249–263. Available at: https://doi.org/10.1016/j.nec.2020.12.005
- Jin Z, MacPherson K, Liu Z & Vu LP (2023). RNA modifications in hematological malignancies. International Journal of Hematology, 117(6), 807-820. Available at: https://doi.org/10.1007/s12185-023-03576-0
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, et al (2016). The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 127(20), 2375-2390. Available at: https://doi.org/10.1182/blood-2016-01-643569
- Jia J, Wang X, Song Z, Meng S, Fei Y, Yu J, Liu X, et al. (2024). A retrospective analysis of mature T- and NK-cell lymphomas. Cancer biology & medicine, 21(3), 223-229. Available at: https://doi.org/10.20892/j.issn.2095-3941.2023.0464
- Ferrand C & Rambaldi A (2022). Myeloid Malignancies. Em The EBMT/EHA CAR-T Cell Handbook (p. 97-103). Springer International Publishing. Available at: https://link.springer.com/chapter/10.1007/978-3-030-94353-0_18#DOI
- Barros LRC, Couto SCF, da Silva Santurio D, Paixão EA, Cardoso F, da Silva VJ, Klinger P, et al. (2022). Systematic review of available CAR-T cell trials around the world. Cancers, 14(11), 2667. Available at: https://doi.org/10.3390/cancers14112667
- Lima MFde, Lisboa MdeO, Terceiro LEL, Rangel-Pozzo A & Mai S (2022). Chromosome territories in hematological malignancies. Cells (Basel, Switzerland), 11(8), 1368. Available at: https://doi.org/10.3390/cells11081368
- Brudno JN & Kochenderfer JN (2019). Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Reviews, 34, 45-55. Available at: https://doi.org/10.1016/j.blre.2018.11.002
- Qi Y, Zhao M, Hu Y, Wang Y, Li P, Cao J, Shi M., et al. (2022). Efficacy and safety of CD19-specific CAR T cell–based therapy in B-cell acute lymphoblastic leukemia patients with CNSL. Blood, 139(23), 3376-3386. Available at: https://doi.org/10.1182/blood.2021013733
- Meng Y, Deng B, Rong L, Li C, Song W, Ling Z, Xu J, et al. (2021). Short-interval sequential CAR-T cell infusion may enhance prior CAR-T cell expansion to augment anti-lymphoma response in B-NHL. Frontiers in oncology, 11. Available at: https://doi.org/10.3389/fonc.2021.640166
- Polyatskin IL, Artemyeva AS & Krivolapov YA (2019). Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition): lymphoid tumors. Arkhiv Patologii, 81(3), 59. Available at: https://doi.org/10.17116/patol20198103159
Correspondence
Patrick Menezes
E-mail: [email protected]
