Laboratory tests for diabetes diagnosis with an emphasis on glucose load testing
Exames laboratoriais para o diagnóstico do diabetes com ênfase nos testes de sobrecarga
Mauren Isfer Anghebem1,2, Clara Helena Zonatto2, Jaqueline Souza Chaves Taniguchi Leite 2, Victor Melgarejo Marques2, Alceu de Oliveira Toledo Júnior3, Fabiane Gomes de Moraes Rego1,4, Geraldo Picheth4
1 Departamento de Análises Clínicas da Universidade Federal do Paraná/UFPR, Curitiba, PR, Brasil.
2 Escola de Medicina e Ciências da Vida da Pontifícia Universidade Católica do Paraná, Curitiba, PR, Brasil.
3 Departamento de Análises Clínicas e Toxicológicas da Universidade Estadual de Ponta Grossa; Diretor Técnico do Laboratório do Hospital Vicentino, Ponta Grossa, PR, Brasil.
4 Programa de Pós-Graduação em Ciências Farmacêuticas da Universidade Federal do Paraná/UFPR, Curitiba, PR, Brasil.
Received on Nov 02, 2024
Approved on Nov 16, 2024
DOI: 10.21877/2448-3877.202400217.en
INTRODUCTION
Diabetes mellitus refers to a heterogeneous group of metabolic disorders caused by insufficient and/or ineffective insulin action, leading to chronic hyperglycemia.(1)
Diabetes is characterized by a prolonged subclinical phase defined by hyperglycemia, which may remain undiagnosed without screening or other early detection mechanisms. In all types of diabetes, the primary goal to minimize the consequences of chronic hyperglycemia is glycemic control.(2) For this reason, clinical laboratory testing is essential for the diagnosis and monitoring of this condition.
Three laboratory tests are used for diagnostic purposes: fasting glucose (FG), random glucose (RG), the oral glucose tolerance test (OGTT), and glycated hemoglobin A1c (HbA1c).(3)
The measurement of fasting plasma glucose after an 8-hour fasting period provides information on glycemic concentration at the time of collection, reflecting glucose regulation in the post-absorptive state, where an increased glucagon/insulin ratio is expected in the plasma. HbA1c reflects the average glycemia over the past 2 to 3 months, assessing long-term glycemic status. The OGTT, in turn, evaluates glucose metabolism and the insulin release response following a controlled glucose load.(4) Each test has advantages and disadvantages that must be carefully considered.
This narrative review, based on a literature search on the PubMed database using descriptors in both English and Portuguese, such as “diabetes,” “diabetes mellitus,” “gestational diabetes,” “diabetes diagnosis,” “glucose tolerance test,” “OGTT,” and “glycemic curve,” aims to provide information on current criteria for diabetes diagnosis, including gestational diabetes, and to highlight the laboratory assays used in diabetes, with an emphasis on glucose load tests, underscoring their differences, purposes, and limitations.
CURRENT CRITERIA FOR THE LABORATORY DIAGNOSIS OF DIABETES
In March 2024, the International Diabetes Federation (IDF) released a position statement consolidating evidence that plasma glucose levels measured one hour after an oral glucose load (OGTT-1h) are more effective for classifying glycemic status compared to isolated FG or HbA1c measurements. Moreover, OGTT-1h facilitates the early detection of individuals at increased risk of progressing to type 2 Diabetes mellitus (T2DM), a condition also known as prediabetes.
Several studies have supported the recommendation of OGTT-1h for the diagnosis of diabetes and prediabetes, as it serves as a more sensitive predictor of T2DM, cardiovascular disease, microangiopathy, and mortality compared to previously used criteria.(5-9) Of particular note is a meta-analysis of 15 studies involving a total of 35,551 participants, including both Caucasian and non-Caucasian ethnic groups (46.2%), such as Amerindians, Japanese, Mexican Americans, and South Asians. This study identified that an OGTT-1h threshold of 209 mg/dL demonstrated good sensitivity and specificity for detecting T2DM. At the cutoff value of 209 mg/dL (95% CI: 10.6, 12.6), a sensitivity of 0.92 (0.87, 0.95), specificity of 0.91 (0.88, 0.93), and an area under the curve (AUC) of 0.939 (95% confidence region for sensitivity, 0.904, 0.946) were observed, along with a positive predictive value of 45%.(10)
Based on these premises, the IDF position statement recommends using OGTT-1h glycemia with validated cutoff points of 155 mg/dL (8.6 mmol/L) for intermediate hyperglycemia and ≥209 mg/dL (11.6 mmol/L) to characterize T2DM. Intermediate hyperglycemia, or “prediabetes,” represents a state between “normal” glycemia and levels diagnostic for diabetes. It encompasses impaired fasting glucose and impaired glucose tolerance.(11)
In Brazil, the current laboratory criteria for the diagnosis of diabetes and prediabetes take this new cutoff into account and are defined as follows: fasting plasma glucose of 126 mg/dL or higher, HbA1c of 6.5% or higher, OGTT-1h glucose of 209 mg/dL or higher, or OGTT-2h glucose of 200 mg/dL or higher. If only one test is abnormal, it should be repeated for confirmation. In the presence of typical hyperglycemia symptoms (polyuria, polydipsia, polyphagia, rapid weight loss, or ketoacidosis), it is recommended that the diagnosis be made with a random plasma glucose measurement of 200 mg/dL or higher (Table 1).(4)
Table 1
Laboratory criteria for the diagnosis of diabetes and prediabetes
| Criteria | Normal | Prediabetes | Diabetes |
| Fasting Glucose * | < 100mg/dL | 100-125mg/dL | ≥ 126mg/dL |
| Random Glucose ** | – | – | ≥ 200mg/dL |
| 1-hour OGTT Glucose # | < 155mg/dL | 155-208mg/dL | ≥ 209mg/dL |
| 2-hour OGTT Glucose # | < 140mg/dL | 140-199mg/dL | ≥ 200mg/dL |
| HbA1c | < 5.7 % | 5.7-6.4% | ≥ 6.5 % |
Legend: OGTT: oral glucose tolerance test; HbA1c: glycated hemoglobin A1c.
*Associated with classic diabetes symptoms.
**Fasting is defined as the cessation of caloric intake for 8 to 12 hours.
Oral load equivalent to 75g of anhydrous glucose diluted in water.
Source: Adapted from Rodacki et al., 2024.(4)
LABORATORY DIAGNOSIS OF GESTATIONAL DIABETES
Gestational Diabetes mellitus (GDM) is defined as carbohydrate intolerance that begins during pregnancy but does not meet the diagnostic criteria for diabetes outside of pregnancy.(12)
The diagnosis of GDM has also evolved over the years to identify hyperglycemia first detected during pregnancy. Two categories can be distinguished during pregnancy: overt diabetes, which is only evident during pregnancy, or GDM.(12)
In 1979, the National Diabetes Data Group (NDDG) recommended conducting a challenge test involving the administration of 50g of glucose and measuring blood glucose after 1 hour during the first prenatal visit for pregnant women with risk factors, known as the O’Sullivan test. This test aimed to diagnose GDM before 24 to 28 weeks of gestation, identifying early-onset gestational diabetes. If the result was positive, a 3-hour OGTT with a 100g glucose load would be performed.(13)
In 2010, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) developed new diagnostic criteria for GDM based on the results of the prospective Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. The IADPSG criteria proposed using a FG value of 92 to 126 mg/dL during the first 24 weeks of pregnancy to define early-onset GDM. This threshold is identical to the one used after 24 weeks of pregnancy.(14)
In 2011, the American Diabetes Association (ADA) and, in 2013, the World Health Organization (WHO) adopted the IADPSG criteria for the diagnosis of GDM.(15) However, WHO noted that if the post-load glucose is ≥ 200 mg/dL, it would be defined as diabetes diagnosed in pregnancy (overt diabetes) rather than GDM.(12)
In 2017, the Brazilian Diabetes Society (SBD), together with the Brazilian Federation of Gynecology and Obstetrics Associations (FEBRASGO), the Pan American Health Organization (PAHO), and the Ministry of Health of Brazil, established a joint proposal for the standardization of GDM screening and diagnosis in the country. The Brazilian criteria were adapted from the WHO criteria,(16) as outlined in the flowchart in Figure 1.
In the first prenatal visit for pregnant women with no prior knowledge of a diabetes diagnosis, it is recommended to perform a FG test to detect overt diabetes and early-onset GDM. The diagnosis of GDM should be considered in pregnant women with FG levels between 92 mg/dL and 125 mg/dL at any point during pregnancy. For all pregnant women without a prior diabetes diagnosis, regardless of risk factors, it is recommended that GDM diagnostic testing be performed between the 24th and 28th weeks of gestation through an OGTT with plasma glucose measurements in fasting, 1 hour, and 2 hours after ingestion of 75g anhydrous glucose. During GDM screening after the 24th week, if the 2-hour glucose level in the 75g OGTT is ≥ 200 mg/dL, the presence of diabetes diagnosed in pregnancy (overt diabetes) should be considered rather than GDM.(16)
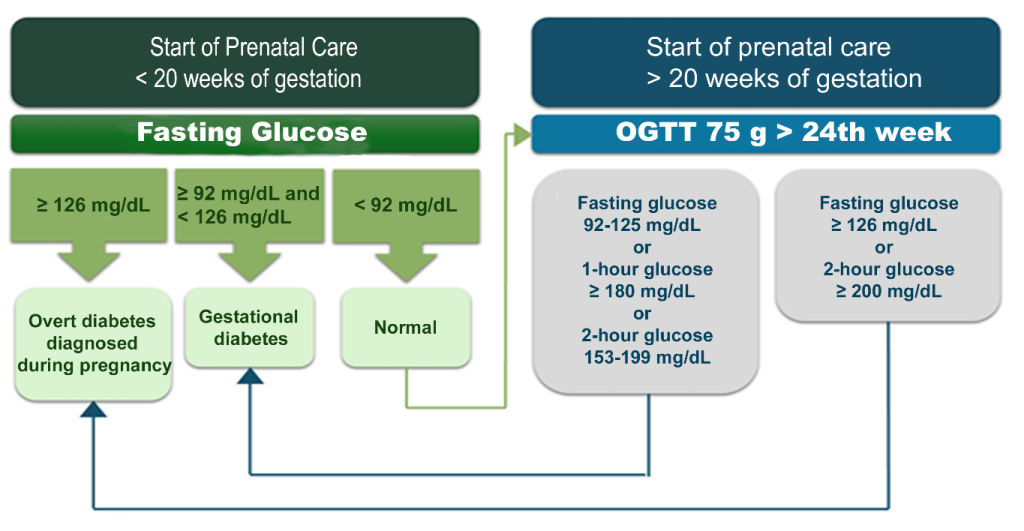
Figure 1
Classification of hyperglycemia in pregnant women without a previously diagnosed diabetes condition.
Legend: DM: Diabetes mellitus; OGTT: oral glucose tolerance test. Source: Adapted from Zajdenverg et al., 2024.(16)
Other strategies are recommended by international organizations, such as the American College of Obstetricians and Gynecologists (ACOG), which endorsed a two-step approach, known as the two-step strategy.(17) In other words, the diagnosis of GDM can be performed using either of the two strategies: 1. One-step strategy: the 75g OGTT derived from the IADPSG criteria, mentioned earlier, or 2. Two-step strategy: an older “two-step” approach with a 50g screening test, without fasting, followed by a 100g OGTT for those with a positive screening result. This strategy is based on the criteria of O’Sullivan and Mahan.(3)
In the one-step strategy, the OGTT is performed with 75g of glucose, and the diagnosis of GDM is made when any of the following plasma glucose values are met or exceeded: fasting plasma glucose ≥ 92mg/dL, 1-hour plasma glucose ≥ 180mg/dL, 2-hour plasma glucose ≥ 153mg/dL. In the two-step strategy, if the 1-hour plasma glucose after the 50g glucose load, without fasting, performed between the 24th and 28th weeks of gestation in individuals without a previous diabetes diagnosis is ≥ 130, 135, or 140mg/dL, a second step is performed; the 100g OGTT after fasting. In this case, the diagnosis of GDM is made when at least two of the following four plasma glucose values (measured fasting, 1, 2, and 3 hours during the OGTT) are met or exceeded: fasting plasma glucose ≥ 95mg/dL, 1-hour plasma glucose ≥ 180mg/dL, 2-hour plasma glucose ≥ 155mg/dL, 3-hour plasma glucose ≥ 140mg/dL.
Since different diagnostic criteria identify varying degrees of maternal hyperglycemia and maternal/fetal risk, there is significant disagreement among specialists regarding which strategy is best for diagnosing GDM.(18-20)
LABORATORY TESTS IN THE CONTEXT OF DIABETES
Each of the laboratory tests recommended for diabetes diagnosis has specific advantages and limitations, as summarized in Table 2.
Table 2
Advantages and limitations of routine laboratory tests for diabetes
| Test | Advantage | Limitation |
| FG | • Simple automated quantification • Low cost • Single sample requirement • Widely available • Established diagnostic criteria |
• Requires ≥ 8 hours of fasting • High biological variability • Diurnal variation • Sample instability (“glycolysis”) • Multiple factors influence glucose concentration (stress, acute illness) • Glucose concentration varies depending on the sample source (venous, capillary, or arterial) • Whole blood glucose concentration differs from plasma levels • FG has a weaker correlation with DM complications (compared to HbA1c) • Reflects glucose homeostasis at a single point in time |
| OGTT | • Sensitive indicator of diabetes risk • Early marker of impaired glucose homeostasis |
• Low reproducibility • Lengthy patient preparation • Time-consuming and inconvenient for the patient • Oral glucose dose may be unpalatable • Higher cost • Influenced by various medications • Subject to the same limitations as FG, such as sample instability • Must be performed in the morning |
| HbA1c | • Does not require patient fasting • Sample can be collected at any time of the day • Low biological variation • Stable sample • Not affected by acute factors such as stress and exercise • Reflects glucose concentration over a long period prior to collection • Standardized assay (NGSP) • Single whole blood sample required • Concentration predicts the development of DM-related microvascular complications • Applicable for guiding treatment decisions |
• Can be affected by factors other than glucose, such as alterations in erythrocyte lifespan and ethnicity • Presence of hemoglobinopathies interferes with the result • May not be available in all laboratories • Higher cost |
Legend: FG: fasting plasma glucose; OGTT: oral glucose tolerance test; HbA1c: glycated hemoglobin A1c; NGSP: National Glycohemoglobin Standardization Program. Source: Adapted from Sacks et al., 2023.(21)
FG demonstrates good reproducibility, is widely available in healthcare services, and its analytical methods are well-established. However, its analysis requires fasting, and values may be influenced by acute conditions and stress, which are common during pediatric collections. Additionally, in samples collected without glycolysis inhibitors such as fluoride or iodoacetate, glucose levels may decrease by 5% to 7% per hour, potentially affecting assay interpretation.(22) Clinical laboratories primarily employ enzymatic methods, with glucose oxidase and hexokinase-UV being the predominant techniques for plasma glucose quantification, both of which are well-established and reproducible. To prevent misclassification of individuals, glucose measurement must minimize total analytical error, and methods should be free of measurable biases. Based on biological variation, glucose measurement should have an analytical imprecision of ≤ 2.4%, a bias of ≤ 2.1%, and a total error of ≤ 6.1%.(21)
HbA1c has notable characteristics, including not requiring fasting and reflecting average blood glucose over the past 2 to 3 months. Among the disadvantages of this test are its significantly higher cost compared to FG and limited availability in some developing countries. Additionally, as an indirect measure of blood glucose, it can be influenced by non-glycemic factors such as hemoglobin variants, medications (e.g., hydroxyurea, vitamin C, aspirin), race, age, renal function, and others.(22) The method used for HbA1c determination must be certified by the National Glycohemoglobin Standardization Program (NGSP, www.ngsp.org) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay. Only in this way are HbA1c results from different laboratories harmonized and comparable to those reported in the DCCT.(3)
Some studies suggest that FG is more accurate than HbA1c for diabetes diagnosis, while HbA1c is more specific but less sensitive when compared to FG and OGTT.(23,24)
FG and HbA1c are the recommended and widely used laboratory tests due to their relative convenience and reproducibility compared to OGTT. The OGTT has the highest sensitivity among the three tests and is considered the gold standard for detecting individuals at increased risk for diabetes development, classified as prediabetes or intermediate hyperglycemia. HbA1c has low sensitivity (47% to 67%) and high specificity (98% to 99%) for diagnosing diabetes, compared to glucose measurement 2 hours after a 75g oral glucose load (OGTT-2h).(4)
The OGTT-2h shows high variability (16.7%) when compared to FG (5.7%) and HbA1c (3.6%).(25) Therefore, among the disadvantages of the OGTT are its low reproducibility, the prolonged time required for the test, and the potential for discomfort (nausea and vomiting) following the glucose load intake.(22)
The accuracy and applicability of laboratory tests for diagnosing hyperglycemia are analyzed through clinical studies, and different strategies and cut-off values have been proposed over time. Thirty years ago, for example, fasting glucose levels of 140 mg/dL or higher were used to diagnose diabetes, and there was no category for increased risk of diabetes, now termed “prediabetes”.(26) Diagnostic criteria for diabetes have also evolved over time to include HbA1c starting in 2010, which had previously only been used for monitoring purposes.(27,28)
Glucose Tolerance Tests
Glucose tolerance assesses the body’s ability to respond to a glucose load.(29) Any test involving the oral intake of a known amount of anhydrous glucose dissolved in water, followed by the determination of plasma glucose at pre-defined time points, is referred to as a glucose tolerance test. In Brazil, the oral glucose tolerance test (OGTT), with measurement of fasting plasma glucose, followed by determination of glucose levels at 1 and 2 hours post-load, is commonly referred to as a glucose tolerance test. However, the measurement of fasting glucose, followed by five or six additional determinations at defined intervals after glucose load, such as at 0 (fasting) followed by collections at 30, 60, 90, 120, and 180 minutes, known as the glucose curve, is also considered a glucose tolerance test.(30) This test is frequently requested by clinicians, although there are no established reference intervals or recommended cut-off criteria in current literature.
OGTT-1h and OGTT-2h
The OGTT was proposed over 100 years ago as a measurement capable of assessing the body’s response to a supraphysiological glucose load.(31) Its use as a tool in the context of diabetes diagnosis has evolved significantly over the last century.(32)
The OGTT remains the reference method for evaluating glucose tolerance, despite its recognized low reproducibility and a high coefficient of variation at the 2-hour mark (OGTT-2h).(33,34) Evidence supports that the OGTT-1h is more effective in classifying glycemic status compared to isolated measurements of fasting glucose or HbA1c. Individuals with intermediate hyperglycemia (prediabetes) and T2DM exhibited increased adiposity, higher blood pressure, elevated uric acid levels, a worse lipid and inflammatory profile, and a progressive reduction in insulin sensitivity compared to normoglycemic individuals. The OGTT-1h can identify individuals with this unfavorable cardiometabolic risk profile.(35)
It should be emphasized that the OGTT is the reference test for diagnosing GDM, where fasting plasma glucose concentrations have reduced diagnostic sensitivity. It is also important to note that, in pregnant women with no prior diagnosis of diabetes, an abnormal OGTT can be an independent risk factor for postpartum hyperglycemia.(17,36)
Glycemic Curve
Serial glucose measurements following a glucose load allow for the observation of a glycemic curve, defined by the pattern of rise and fall in glucose concentrations after the glucose load. The shape of the curve thus reflects pancreatic beta-cell function and metabolic risk.(29) Differences in the shape of the glycemic curve have been documented since the 1950s, but only recently have researchers considered using the characteristics of the glucose curve as a predictive tool.(37)
Studies have shown that the shape of the glucose curve can be used to predict glucose intolerance and the risk of progression to T2DM.(30,38) The glycemic curve profile is defined as monophase, biphasic, or triphasic, or unclassified, as depicted in Figure 2. A monophase curve can be defined as an increase in glucose between 30 and 90 minutes, followed by a decline between 90 and 120 minutes. A biphasic response curve is characterized by a decrease after an initial increase, followed by a second increase.(39) The biphasic pattern is found in 20% to 30% of adults without diabetes.(38)
Individuals with a monophase curve have lower insulin sensitivity and decreased beta-cell function compared to those with a biphasic curve.(40,41) A biphasic or triphasic (unclassified) glycemic response profile, characterized by an increase, a decrease, and a subsequent rise in glucose following the glucose load, has been associated with better beta-cell function and lower glucose concentrations compared to a monophase pattern.(37,41-43) A multi-ethnic cohort of adults with recent diagnoses of T2DM compared differences in the shape of the glycemic curve, considering sex, race, body mass index, and metabolic differences between the two most common curve types: monophase and continuous increase (where glucose concentration only rises during the test period). The group that showed a continuous increase in glucose was associated with greater beta-cell dysfunction and higher HbA1c values compared to the group with a monophase pattern. These data suggest that the shape of the curve could also serve as a biomarker for diabetes.(29)
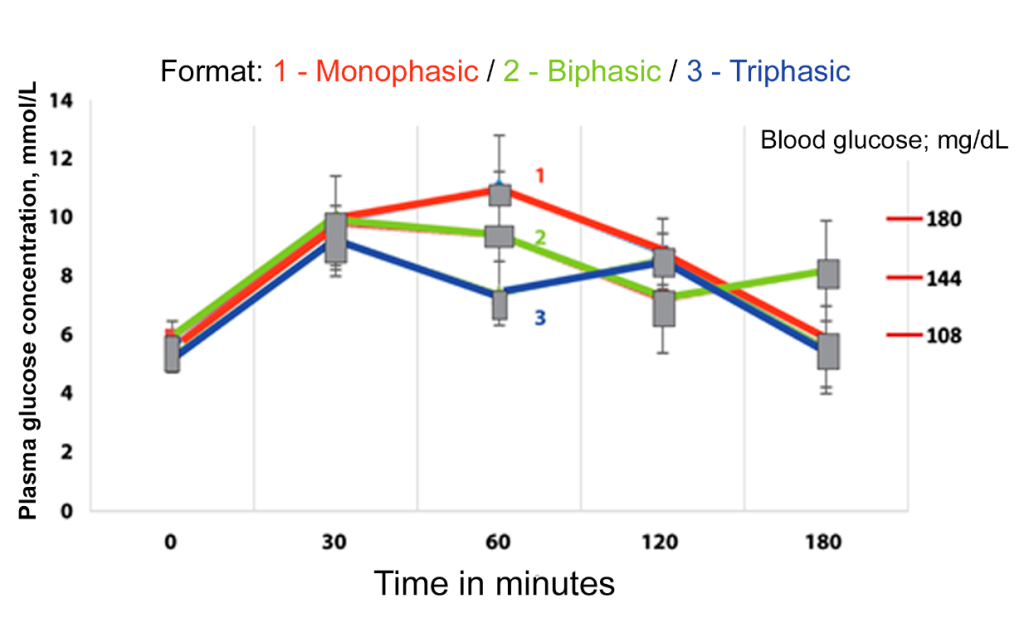
Figure 2
Patterns of glycemic curve shape.
Legend: The y-axis, numbered from 0 to 14, represents plasma glucose concentration in mmol/L. The x-axis, numbered from 0 to 180, represents time in minutes. The blue line represents a monophase glycemic curve pattern. The red line represents a biphasic glycemic curve pattern. The green line represents a triphasic glycemic curve pattern. Source: Adapted from Cheng et al., 2019.(37)
FACTORS INFLUENCING THE ORAL GLUCOSE TOLERANCE TEST
The performance of the OGTT is directly affected by pre-analytical and analytical factors, as well as those related to laboratory glucose measurement. The reproducibility of the OGTT has been questioned for decades, but it continues to be considered the “gold standard” for the diagnosis of T2DM and GDM.(44-46)
Pre-analytical factors such as biological or intraindividual variation, gastric emptying, the glucose solution for the overload from different manufacturers, the release of hyperglycemic hormones like cortisol and adrenaline, age, and sex can affect the reproducibility of the OGTT;(47) as well as analytical factors such as analyzer calibration, the method, and the reagents used in the determination of plasma glucose.(47,48) These variables contribute to the OGTT having a reproducibility of 64% to 80%.(47,49)
Below, we present and discuss some factors that influence this test.
Diet
Proper preparation for the OGTT is essential to prevent falsely elevated results due to low carbohydrate intake in the days preceding the test. The mechanisms by which low-carbohydrate diets affect glucose metabolism are complex and not fully understood. Some propose that the mechanism is partly due to the loss of the first phase of insulin release, resulting in reduced peripheral and hepatic glucose uptake and incomplete suppression of hepatic glucose production. Low carbohydrate intake also impacts insulin sensitivity and is associated with an increase in free fatty acids in the plasma, which may reduce insulin secretion.(50)
Some studies have shown that a low-carbohydrate diet would not affect the outcome of the OGTT and that maintaining the usual diet before undergoing the test would better reflect an individual’s ability to metabolize glucose.(51,52) However, to maintain a standardized approach and enhance the reproducibility of the method, the OGTT should be preceded by a diet without carbohydrate restrictions, with a minimum daily intake of 150g of carbohydrates during the 3 days preceding the test, which should be performed after an 8 to 10-hour fast.(3,53)
Physical Exercise
Physical exercise can alter the results of laboratory tests. Despite contradictory data in the literature regarding the duration and intensity of exercise that would affect test outcomes, physical activity influences how the body processes nutrients, such as glucose.(54-56)
At the onset of physical activity, there is an inhibition of insulin secretion and, consequently, hyperglycemia. This mechanism occurs to provide energy for the body. An elevation in plasma glucose can be observed up to 4 hours after strenuous exercise, such as a marathon, returning to baseline within 24 hours.(57)
After this initial phase of hyperglycemia, glucose is taken up by muscle cells independently of insulin, likely due to an increase in the number of active membrane transporters, leading to hypoglycemia. The prolonged reduction in glucose concentration is influenced by adrenaline – an antagonist of insulin – which ceases to be released after exhaustive physical exertion, causing the effects of insulin to become predominant and amplifying hypoglycemia for approximately 2 hours after the physical activity ends.(58) Therefore, it is recommended that strenuous physical exercise be avoided in the 24 hours prior to performing the OGTT.
Smoking
Another interfering factor of the OGTT is tobacco use. Smoking acutely impairs glucose tolerance and insulin sensitivity; therefore, smoking is not permitted during the OGTT.(59-61)
Glucose Solution
The composition of the oral glucose solution, including added excipients to enhance taste and odor, can impact endogenous insulin secretion and, consequently, plasma glucose concentrations.(48) For this reason, standardizing and validating the glucose solution is advisable.
Most institutions conducting the OGTT use a commercially available liquid solution containing 75g of pure D-glucose dissolved in 300mL of water, due to its convenience, commercial preparation quality standards, and the availability of flavored options to minimize discomfort. Human perception of sweetness can be altered by temperature, which supports the recommendation to administer the glucose solution cold rather than at room temperature to reduce nausea, particularly in pregnant women.(62)
If the establishment is preparing the glucose solution in-house, it must follow good laboratory practices to ensure that the final solution is at the standardized concentration. Carbonated water appears to mitigate the unpleasant side effects of the glucose solution, but it affects OGTT results by promoting a higher post-load glucose level at 1 hour compared to tests conducted with non-carbonated water. Therefore, carbonated water should not be used.(62)
In the case of OGTT in children or individuals with low body weight, it is recommended to administer 1.75g of glucose per kg of body weight, up to a maximum of 75g. For example, a 12kg child should consume 84mL of the 75g glucose solution, as illustrated in Figure 3.

Figure 3: Calculation of the glucose solution volume for children and individuals with low body weight.
Source: Authors.
Gastric emptying
The absorption behavior of the glucose solution shows intra- and interindividual variability, also impacting the variability of glucose concentrations in the OGTT.(1) This is because the rate of gastric emptying has high individual variability and is a factor that affects plasma glucose concentration. The glucose load administered during the OGTT can only pass into the bloodstream after being emptied from the stomach, digested into monosaccharides, and transported through the intestinal epithelium. The transport capacity of the small and large intestines exceeds the 75g of glucose provided during the OGTT; therefore, a limiting step in the rate of glucose absorption is the gastric emptying rate.(63) In other words, gastric emptying is one of the main factors influencing the glycemic response in the first hour after the OGTT or a meal and is responsible for 30% to 35% of the variability in postprandial blood glucose.(63)
To minimize the impact of gastric emptying on the OGTT results, the total volume of the glucose solution should be consumed, preferably, within 5 minutes.(1)
Emesis
Gastric intolerance to the glucose solution has been linked to the high osmolarity of the solution, caused by the high concentration of glucose, which also delays gastric emptying. Emesis is the primary reason for the failure to complete the OGTT;(65) therefore, it may be suggested that the individual take an antiemetic before undergoing the test.(64)
In cases of emesis, the test should be interrupted and rescheduled. However, if the individual vomits after 30 minutes of ingesting the solution, some laboratories may proceed with the OGTT, noting in the report’s observation that the patient vomited, how many times emesis occurred, and how many minutes after ingestion it happened.
It should be considered that hyperemesis gravidarum causes changes in maternal metabolism during the first trimester of pregnancy due to limited caloric intake and fasting. This can reduce the positive predictive value of gestational diabetes screening in the first trimester through the OGTT, leading to an increase in false-positive results. In such cases, it may be beneficial to consider other screening and diagnostic strategies.(66,67)
Medication interference
Interpreting the OGTT can become challenging for individuals taking high doses of medications known to induce hyperglycemia, such as glucocorticoids, calcium channel blockers, oral contraceptives, protease inhibitors, interferon, beta-blockers, diuretics, among others.(68)
Type of sample
Blood glucose can be quantified in whole blood, serum, or plasma, but plasma is the recommended sample type for diagnosis. It should be noted that the water content in plasma is approximately 11% higher compared to whole blood, which results in plasma glucose being approximately 11% higher than whole blood glucose in individuals with normal hematocrit.(63)
Glucose concentrations during an OGTT in capillary blood from fingertip puncture are significantly higher than those in venous blood (an average of 30 mg/dL, equivalent to 20% to 25%), likely due to glucose consumption by tissues. In contrast, the average difference in fasting samples is only 2 mg/dL. That is, plasma and capillary glucose concentrations are comparable in the fasting state, but post-load capillary glucose is significantly higher than venous sample concentrations.(69)
Although there are studies on the feasibility of self-collecting capillary blood using specific devices for OGTTs,(70-72) venous blood samples have been the recommended standard.(73)
Collection Tube
The rate of glucose consumption/reduction in a sample is 5% to 7% per hour, making in vitro glycolysis a pre-analytical interference with the potential to alter the interpretation of the OGTT. To minimize the effect of glycolysis, two strategies can be employed by the laboratory: 1. Blood collection in a tube without a glycolytic inhibitor, followed by immediate or within 30 minutes after collection centrifugation and serum separation from blood cells; 2. Blood collection in tubes containing a glycolytic inhibitor.(21,69,74)
Thus, in locations where immediate sample processing is not possible, the use of glycolysis inhibitors, such as sodium fluoride (NaF; 2.5 mg of fluoride/mL of blood), is recommended. However, NaF alone is not a rapid-acting glycolysis inhibitor.(21) The mechanism of action of fluoride is based on the inhibition of the enzyme enolase, which acts later in the glycolytic pathway. Consequently, the activity of glycolytic enzymes upstream of enolase is not significantly affected, and these enzymes remain active, metabolizing glucose. This explains why the full effect of fluoride in inhibiting glycolysis may take between 30 minutes and 4 hours, during which the glucose concentration in the collection tube can decrease considerably, especially when the sample is stored at room temperature.(75) After 4 hours, glucose concentration in whole blood remains stable for up to 72 hours at room temperature in the presence of fluoride.(21,74)
To enhance glycolytic action, NaF can be used in combination with anticoagulants such as potassium oxalate, EDTA, citrate, or lithium heparin. Several studies have demonstrated the efficacy of tubes containing citrate/fluoride/EDTA (CFE) in inhibiting glycolysis,(76-78) and currently, the use of citrate-buffered collection tubes is recommended for glucose determination, especially if sample processing is likely to take longer than 30 minutes after collection.(21)
It is also important to consider that, in samples with high white blood cell counts, glycolysis can increase even in the presence of fluoride.(21)
Glucose Measurement
The measurement of glucose is the central analytical factor in the OGTT. An inaccurate glucose measurement can lead to diagnostic errors, incorrect patient management, unfavorable outcomes, and increased healthcare costs. For instance, glucose measured from heparinized plasma is approximately 5% lower than glucose measured from serum, possibly due to the fluid shift from erythrocytes to plasma caused by the anticoagulant.(69)
Glucose measurement methods should be calibrated (traceable) to reference methods. Currently, there are two reference methods for measuring plasma glucose recommended by the Joint Committee for Traceability in Laboratory Medicine: isotope dilution mass spectrometry (IDMS) and the hexokinase/glucose-6-phosphate dehydrogenase enzymatic method. The maximum allowable deviation between the laboratory method and the reference method is 4%. In laboratory settings, glucose is commonly determined using one of the following enzymatic methods: hexokinase, glucose dehydrogenase, or glucose oxidase in reactions coupled to a chromophore, ultraviolet absorption, or generating an electrical current.(63)
Methodologies using glucose oxidase/peroxidase (colorimetric) and UV hexokinase (ultraviolet) show similar results and do not affect the characterization of Diabetes mellitus.(79)
FINAL CONSIDERATIONS
Diabetes is a chronic, multifactorial, and silent syndrome affecting approximately 10% of the global population. Without a cure, it is crucial that DM diagnosis be early to minimize chronic vascular complications.
Clinical laboratories play a central role in the screening, diagnosis, and monitoring of DM through plasma glucose, HbA1c, and OGTT. Recently, glucose measurement 1 hour after oral glucose load (OGTT-1h) has been incorporated into the criteria for diagnosing diabetes and pre-diabetes, as it is a more sensitive and earlier predictor of T2DM, cardiovascular disease, microangiopathy, and mortality compared to previously used criteria. OGTT-1h values of 155 mg/dL indicate intermediate hyperglycemia (prediabetes), while values ≥ 209 mg/dL classify as diabetes.
Thus, the glucose load test gains new prominence. It is important to emphasize that any test performed after oral ingestion of a known amount of glucose, followed by plasma glucose measurement, constitutes a glucose load test. In Brazil, fasting plasma glucose measurement followed by glucose determination 1 hour and 2 hours after glucose solution ingestion is commonly referred to as OGTT. Meanwhile, the glucose load test involving fasting glucose determination followed by 5 or 6 glucose measurements every 30 minutes after glucose solution ingestion is known as the glucose curve.
For conducting glucose load tests, it is recommended to avoid intense physical exercise within 24 hours prior to the test. Smoking is not permitted during the procedure. The test is performed after an 8- to 10-hour fast, and during the three days preceding the test, a diet including at least 150 g of carbohydrates per day is recommended.
The glucose solution should contain 75 g of glucose dissolved in 250–300 mL of water, or 1.75 g of glucose per kilogram, up to a maximum of 75 g. The total volume of the glucose solution should ideally be ingested within 5 minutes. In case of emesis, the test must be interrupted and rescheduled. However, if the patient vomits after 30 minutes of ingestion, continuing the test may be considered, with the occurrence duly reported in the test report.
It is preferable to use plasma collected in tubes containing citrate/fluoride/EDTA for the OGTT if centrifugation and plasma separation from blood cells cannot be performed within 30 minutes of collection.
The methodology used for glucose measurement should be traceable to reference methods, with enzymatic colorimetric methods employing glucose oxidase/peroxidase or UV enzymatic methods utilizing hexokinase being acceptable options.
Finally, it is important to emphasize that OGTT and the glucose curve exhibit significant variability, which may affect result interpretation. This study reviews observations on glucose load tests and highlights the new criteria for diabetes diagnosis.
REFERENCES
- Pleus S, Tytko A, Landgraf R, Heinemann L, Werner C, Müller-Wieland D, et al. Definition, Classification, Diagnosis and Differential Diagnosis of Diabetes Mellitus: Update 2023. Experimental and Clinical Endocrinology & Diabetes. 2024 Mar 20;132(03):112-24.
- Zhou B, Sheffer KE, Bennett JE, Gregg EW, Danaei G, Singleton RK, et al. Global variation in diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c. Nat Med. 2023 Nov 9;29(11):2885-901.
- ElSayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, Ekhlaspour L, et al. ADA. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024 Jan 1;47(Supplement_1):S20-42.
- Rodacki M, Cobas RA, Zajdenverg L, Silva Júnior WS da, Giacaglia L, Calliari LE, et al. Diagnóstico de diabetes mellitus. In: Diretriz da Sociedade Brasileira de Diabetes. Conectando Pessoas; 2024.
- Abdul-Ghani MA, Abdul-Ghani T, Ali N, DeFronzo RA. One-Hour Plasma Glucose Concentration and the Metabolic Syndrome Identify Subjects at High Risk for Future Type 2 Diabetes. Diabetes Care. 2008 Aug 1;31(8):1650-5.
- Ha J, Chung ST, Bogardus C, Jagannathan R, Bergman M, Sherman AS. One-hour glucose is an earlier marker of dysglycemia than two-hour glucose. Diabetes Res Clin Pract. 2023 Sep;203:110839.
- Alyass A, Almgren P, Akerlund M, Dushoff J, Isomaa B, Nilsson P, et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia. 2015 Jan 8;58(1):87-97.
- Fiorentino TV, Marini MA, Andreozzi F, Arturi F, Succurro E, Perticone M, et al. One-Hour Postload Hyperglycemia Is a Stronger Predictor of Type 2 Diabetes Than Impaired Fasting Glucose. J Clin Endocrinol Metab. 2015 Oct 1;100(10):3744-51.
- Fiorentino TV, Marini MA, Succurro E, Andreozzi F, Perticone M, Hribal ML, et al. One-Hour Postload Hyperglycemia: Implications for Prediction and Prevention of Type 2 Diabetes. J Clin Endocrinol Metab. 2018 Sep 1;103(9):3131-43.
- Ahuja V, Aronen P, Pramodkumar TA, Looker H, Chetrit A, Bloigu AH, et al. Erratum. Accuracy of 1-Hour Plasma Glucose During the Oral Glucose Tolerance Test in Diagnosis of Type 2 Diabetes in Adults: A Meta-analysis. Diabetes Care 2021;44:1062–1069. Diabetes Care. 2021 Jun;44(6):1457-1457.
- Bergman M, Manco M, Satman I, Chan J, Schmidt MI, Sesti G, et al. International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res Clin Pract. 2024 Mar;209:111589.
- Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res Clin Pract. 2014 Mar;103(3):341-63.
- Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes. 1979 Dec 1;28(12):1039-57.
- International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care. 2010 Mar 1;33(3):676-82.
- González‐González NL, González‐Dávila E, Megía A, Pintado P, Vega B, Padrón E, et al. The NDDG criteria versus the IADPSG or the ADA criteria for diagnosing early‐onset gestational diabetes mellitus or abnormal glucose tolerance. International Journal of Gynecology & Obstetrics. 2023 Mar 26;160(3):906-14.
- Zajdenverg L, Façanha CFS, Dualib PM, Golbert A, Moisés ECD, Calderon I de MP, et al. Rastreamento e diagnóstico da hiperglicemia na gestação. In: Diretriz Oficial da Sociedade Brasileira de Diabetes. Conectando Pessoas; 2022.
- Mi C, Liu H, Peng H, Cheng C, Wang M, Liu H, et al. Relationships Among Pre-Pregnancy BMI, Gestational, and Postpartum Oral Glucose Tolerance Results in Women With Gestational Diabetes Mellitus. Front Nutr. 2021 Dec 1;8.
- Brown FM, Wyckoff J. Application of One-Step IADPSG Versus Two-Step Diagnostic Criteria for Gestational Diabetes in the Real World: Impact on Health Services, Clinical Care, and Outcomes. Curr Diab Rep. 2017 Oct 10;17(10):85.
- Moon JH, Jang HC. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab J. 2022 Jan 31;46(1):3-14.
- Ramezani Tehrani F, Sheidaei A, Rahmati M, Farzadfar F, Noroozzadeh M, Hosseinpanah F, et al. Various screening and diagnosis approaches for gestational diabetes mellitus and adverse pregnancy outcomes: a secondary analysis of a randomized non-inferiority field trial. BMJ Open Diabetes Res Care. 2023 Dec 12;11(6):e003510.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, et al. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care. 2023 Oct 1;46(10):e151-99.
- Garonzi C, Maguolo A, Maffeis C. Pros and Cons of Current Diagnostic Tools for Risk-Based Screening of Prediabetes and Type 2 Diabetes in Children and Adolescents with Overweight or Obesity. Horm Res Paediatr. 2023;96(4):356-65.
- Duong KNC, Tan CJ, Rattanasiri S, Thakkinstian A, Anothaisintawee T, Chaiyakunapruk N. Comparison of diagnostic accuracy for diabetes diagnosis: A systematic review and network meta-analysis. Front Med (Lausanne). 2023 Jan 24;10.
- Kaur G, Lakshmi PVM, Rastogi A, Bhansali A, Jain S, Teerawattananon Y, et al. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: A systematic review and meta-analysis. PLoS One. 2020 Nov 20;15(11):e0242415.
- Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term Variability in Measures of Glycemia and Implications for the Classification of Diabetes. Arch Intern Med. 2007 Jul 23;167(14):1545.
- Wareham NJ, O’Rahilly S. The changing classification and diagnosis of diabetes. BMJ. 1998 Aug 8;317(7155):359-60.
- International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009 Jul 1;32(7):1327-34.
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010 Jan 1;33(Supplement_1):S62-9.
- Utzschneider KM, Younes N, Rasouli N, Barzilay JI, Banerji MA, Cohen RM, et al. Shape of the OGTT glucose response curve: relationship with β-cell function and differences by sex, race, and BMI in adults with early type 2 diabetes treated with metformin. BMJ Open Diabetes Res Care. 2021 Sep 16;9(1):e002264.
- Vejrazkova D, Vankova M, Lukasova P, Hill M, Vcelak J, Tura A, et al. The Glycemic Curve during the Oral Glucose Tolerance Test: Is It Only Indicative of Glycoregulation? Biomedicines. 2023 Apr 25;11(5):1278.
- Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of Glycemia for the Diagnosis of Type 2 Diabetes Mellitus. Ann Intern Med. 2002 Aug 20;137(4):263.
- Jagannathan R, Neves JS, Dorcely B, Chung ST, Tamura K, Rhee M, et al. The Oral Glucose Tolerance Test: 100 Years Later. Diabetes Metab Syndr Obes. 2020 Oct;Volume 13:3787-805.
- Feskens E, Bowles C, Kromhout D. Intra- and interindividual variability of glucose tolerance in an elderly population. J Clin Epidemiol. 1991;44(9):947-53.
- Chai JH, Ma S, Heng D, Yoong J, Lim WY, Toh SA, et al. Impact of analytical and biological variations on classification of diabetes using fasting plasma glucose, oral glucose tolerance test and HbA1c. Sci Rep. 2017 Oct 20;7(1):13721.
- Cefalo CMA, Riccio A, Fiorentino TV, Succurro E, Mannino GC, Perticone M, et al. Pathophysiological characteristics of subjects with intermediate hyperglycemia and type 2 diabetes identified by 1-hour plasma glucose during an oral glucose tolerance test. Diabetes Res Clin Pract. 2024 Nov;217:111856.
- Song G, Wei Y, Juan J, Niu J, Yang H. The predictive ability of the triglyceride glucose index, fasting glucose and oral glucose tolerance test for postpartum hyperglycemia in women with a GDM history. The Journal of Maternal-Fetal & Neonatal Medicine. 2024 Jan 2;37(1).
- Cheng X, Yang N, Li Y, Sun Q, Qiu L, Xu L, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds β–cell function in a large Chinese population. BMC Endocr Disord. 2019 Dec 5;19(1):119.
- Abdul‐Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev. 2010 May 20;26(4):280-6.
- Ismail HM, Xu P, Libman IM, Becker DJ, Marks JB, Skyler JS, et al. The shape of the glucose concentration curve during an oral glucose tolerance test predicts risk for type 1 diabetes. Diabetologia. 2018 Jan 27;61(1):84-92.
- Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the Shape of the Glucose Curve During an Oral Glucose Tolerance Test. Diabetes Care. 2003 Apr 1;26(4):1026-33.
- Kim JY, Michaliszyn SF, Nasr A, Lee S, Tfayli H, Hannon T, et al. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test Heralds Biomarkers of Type 2 Diabetes Risk in Obese Youth. Diabetes Care. 2016 Aug 1;39(8):1431-9.
- de Andrade Mesquita L, Pavan Antoniolli L, Cittolin-Santos GF, Gerchman F. Distinct metabolic profile according to the shape of the oral glucose tolerance test curve is related to whole glucose excursion: a cross-sectional study. BMC Endocr Disord. 2018 Dec 16;18(1):56.
- Arslanian S, El ghormli L, Young Kim J, Bacha F, Chan C, Ismail HM, et al. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test: Forerunner of Heightened Glycemic Failure Rates and Accelerated Decline in β-Cell Function in TODAY. Diabetes Care. 2019 Jan 1;42(1):164-72.
- Lages M, Barros R, Moreira P, Guarino MP. Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review. Nutrients. 2022 May 12;14(10):2032.
- Kirke AB, Spry E, Atkinson D, Sinclair C, Marley J V. Oral glucose tolerance test—The imperfect gold standard for gestational diabetes screening: A qualitative study involving clinicians in regional, rural and remote areas of Western Australia. Health Promotion Journal of Australia. 2024 Jul 7.
- Gomez P, Sanchez J. Type 1 Diabetes Screening and Diagnosis. Endocrinol Metab Clin North Am. 2024 Mar;53(1):17-26.
- Testa R, Lo Cascio C, Fabbietti P, Bonfigli AR. OGTT reproducibility in adults with impaired fasting glucose is nearly 65% with adoption of Italian SIBioC-SIPMeL recommendations. Clinical Chemistry and Laboratory Medicine (CCLM). 2021 Jul 27;59(8):e341-3.
- Heinemann L. Are all glucose solutions used for oGTT equal? Diabetic Medicine. 2022 May 7;39(5).
- Jagannathan R, DuBose CW, Mabundo LS, Chung ST, Ha J, Sherman A, et al. The OGTT is highly reproducible in Africans for the diagnosis of diabetes: Implications for treatment and protocol design. Diabetes Res Clin Pract. 2020 Dec;170:108523.
- Klein KR, Walker CP, McFerren AL, Huffman H, Frohlich F, Buse JB. Carbohydrate Intake Prior to Oral Glucose Tolerance Testing. J Endocr Soc. 2021 May 1;5(5).
- Secen EI, Desdicioglu R, Ergun GT, Usta E, Ozgu-Erdinc AS. The Relationship between a High Carbohydrate Diet and Oral Glucose Tolerance Test in Pregnancy. Z Geburtshilfe Neonatol. 2024 Jun 17;228(03):255-9.
- Buhling KJ, Elsner E, Wolf C, Harder T, Engel B, Wascher C, et al. No influence of high- and low-carbohydrate diet on the oral glucose tolerance test in pregnancy. Clin Biochem. 2004 Apr;37(4):323-7.
- Rosenberg EA, Seely EW, James K, Arenas J, Callahan MJ, Cayford M, et al. Relationship between carbohydrate intake and oral glucose tolerance test results among pregnant women. Diabetes Res Clin Pract. 2021 Jun;176:108869.
- Romagnoli M, Alis R, Aloe R, Salvagno GL, Basterra J, Pareja-Galeano H, et al. Influence of training and a maximal exercise test in analytical variability of muscular, hepatic, and cardiovascular biochemical variables. Scand J Clin Lab Invest. 2014 Apr 31;74(3):192-8.
- Sanchis-Gomar F, Lippi G. Physical activity – an important preanalytical variable. Biochem Med (Zagreb). 2014;68-79.
- Fragala MarenS, Bi C, Chaump M, Kaufman HW, Kroll MH. Associations of aerobic and strength exercise with clinical laboratory test values. PLoS One. 2017 Oct 23;12(10):e0180840.
- Foran SE, Lewandrowski KB, Kratz A. Effects Of Exercise On Laboratory Test Results. Lab Med. 2003 Oct 1;34(10):736-42.
- Hughes DC, Ellefsen S, Baar K. Adaptations to Endurance and Strength Training. Cold Spring Harb Perspect Med. 2018 Jun;8(6):a029769.
- Frati AC, Iniestra F, Ariza CR. Acute Effect of Cigarette Smoking on Glucose Tolerance and Other Cardiovascular Risk Factors. Diabetes Care. 1996 Feb 1;19(2):112-8.
- Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016 May 4;12(5):299-308.
- Grøndahl MF, Bagger JI, Lund A, Faurschou A, Rehfeld JF, Holst JJ, et al. Effects of Smoking Versus Nonsmoking on Postprandial Glucose Metabolism in Heavy Smokers Compared With Nonsmokers. Diabetes Care. 2018 Jun 1;41(6):1260-7.
- Wang P, Chang PC, Wang CY, Wang LC, Shih CL. Comparing the effects of water temperature and additives in glucose solution on pregnant women’s taste, side effects, and glycemic levels during an oral glucose tolerance test: a randomized controlled trial. Am J Obstet Gynecol MFM. 2023 Apr;5(4):100870.
- Bogdanet D, O’Shea P, Lyons C, Shafat A, Dunne F. The Oral Glucose Tolerance Test—Is It Time for a Change?—A Literature Review with an Emphasis on Pregnancy. J Clin Med. 2020 Oct 27;9(11):3451.
- Navarro-Martinez H, Flores-Le Roux JA, Llauradó G, Gortazar L, Payà A, Mañé L, et al. One abnormal value or vomiting after oral glucose tolerance test in pregnancy: incidence and impact on maternal-fetal outcomes. Gynecological Endocrinology. 2023 Dec 14;39(1).
- Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: problems associated with the oral glucose tolerance test. Diabetes Res Clin Pract. 2004 Jan;63(1):73-4.
- Madendag Y, Sahin E, Madendag Col I, Eraslan SM, Tayyar AT, Ozdemir F, et al. The effect of hyperemesis gravidarum on the 75 g oral glucose tolerance test screening and gestational diabetes mellitus. The Journal of Maternal-Fetal & Neonatal Medicine. 2018 Aug 3;31(15):1989-92.
- Bayraktar B, Balıkoğlu M, Bayraktar MG, Kanmaz AG. The Effects of Hyperemesis Gravidarum on the Oral Glucose Tolerance Test Values and Gestational Diabetes. Prague Med Rep. 2021;122(4):285-93.
- Fathallah N, Slim R, Larif S, Hmouda H, Ben Salem C. Drug-Induced Hyperglycaemia and Diabetes. Drug Saf. 2015 Dec 14;38(12):1153-68.
- Kuwa K, Nakayama T, Hoshino T, Tominaga M. Relationships of glucose concentrations in capillary whole blood, venous whole blood and venous plasma. Clinica Chimica Acta. 2001 May;307(1-2):187-92.
- Bethel MA, Price HC, Sourij H, White S, Coleman RL, Ring A, et al. Evaluation of a Self-Administered Oral Glucose Tolerance Test. Diabetes Care. 2013 Jun 1;36(6):1483-8.
- Dunseath GJ, Bright D, Jones C, Dowrick S, Cheung W ‐Y., Luzio SD. Performance evaluation of a self‐administered home oral glucose tolerance test kit in a controlled clinical research setting. Diabetic Medicine. 2019 Jul 26;36(7):862-7.
- Tan AYS, Tan MS, Wu A, Seah AC, Chong C, Koh E, et al. Self-administered oral glucose tolerance test with capillary glucose measurements for the screening of diabetes mellitus in high-risk adults: a feasibility study. BMJ Open Diabetes Res Care. 2021 Dec 24;9(2):e002556.
- Larsson-Cohn U. Differences between capillary and venous blood glucose during oral glucose tolerance tests. Scand J Clin Lab Invest. 1976 Dec;36(8):805-8.
- Jung J, Garnett E, Rector K, Jariwala P, Devaraj S. Effect of Collection Tube Type on Glucose Stability in Whole Blood. Ann Clin Lab Sci. 2020 Jul;50(4):557-9.
- Lippi G, Nybo M, Cadamuro J, Guimaraes JT, van Dongen-Lases E, Simundic AM. Blood Glucose Determination: Effect of Tube Additives. In 2018. p. 101-23.
- Daly N, Flynn I, Carroll C, Stapleton M, O’Kelly R, Turner MJ. Comparison of Citrate-Fluoride-EDTA with Fluoride-EDTA Additives to Stabilize Plasma Glucose Measurements in Women Being Screened during Pregnancy with an Oral Glucose Tolerance Test: A Prospective Observational Study. Clin Chem. 2016 Jun 1;62(6):886-7.
- Fobker M. Stability of glucose in plasma with different anticoagulants. Clinical Chemistry and Laboratory Medicine (CCLM). 2014 Jan 1;52(7).
- Pleus S, Beil A, Baumstark A, Haug C, Freckmann G. Plasma Glucose Concentrations in Different Sampling Tubes Measured on Different Glucose Analysers. Experimental and Clinical Endocrinology & Diabetes. 2024 May 2;132(05):260-6.
- Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Ehlers GW, et al. State of the Art in Trueness and Interlaboratory Harmonization for 10 Analytes in General Clinical Chemistry. Arch Pathol Lab Med. 2008 May 1;132(5):838-46.
Correspondence
Mauren Isfer Anghebem
E-mail: [email protected]
