Functional coprological examination
Exame coprológico funcional
Lenilza Mattos Lima1, Vera Lucia Pagliusi Castilho2
1 Universidade Federal de Santa Catarina. Florianópolis, SC, Brasil.
2 Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo – HCFMUSP, Divisão de Laboratório Central – Parasitologia Clínica. São Paulo, SP, Brasil.
Received on Oct 09, 2024
Approved on Oct 23, 2024
DOI: 10.21877/2448-3877.202400215.en
INTRODUCTION
The digestive system is one of the most important systems in the human body. It is responsible for the processing and absorption of nutrients from ingested food, enabling the proper functioning of the organism. For the intestines to function adequately, a set of habits is necessary, including a good intake of fiber (fruits, vegetables, legumes, and cereals) and water.(1,2)
The presence of fiber in stool helps promote proper intestinal function, preventing constipation and fostering bowel regularity. Furthermore, the intestinal bacteria present in fecal matter play a significant role in maintaining the balance of the gut microbiota. The appearance and consistency of the fecal matter can vary according to diet, hydration, and the individual’s health status.(1,2)
The consistency of stool is directly related to its water content. Typically, stool has the following composition:(1)
Water ………………………………….. 75%
Solid matter ………………………….. 25%
Dead bacteria ……………………….. 30%
Fat ………………………………………….. 10% to 20%
Inorganic matter ………………….. 10% to 20%
Proteins ………………………………… 2% to 3%
Undigested residue ……………… 30%
The purpose of functional coprology is to study the motor, digestive, and absorptive functions of the digestive tract through stool analysis. The study encompasses macroscopic and microscopic digestibility tests, as well as chemical examinations. Fecal fat measurement is requested separately, as they require a distinct prescribed diet. Stool parasitology and stool culture are complementary examinations and are requested independently.(1-3)
The results of the functional coprological examination can guide the clinician in diagnosing potential gastrointestinal functional disorders, establishing coprological syndromes such as the following: gastric insufficiency, pancreatic insufficiency, biliary insufficiency, putrefaction, hydrogen fermentation, fecal and ileal syndrome, constipation, colitis, and intestinal disaccharidase deficiency.(1,2)
The role of the clinical analysis laboratory is highly relevant in stool analysis, interpreting and releasing results that will assist the physician in coprological syndromes.
The literature contains few publications on functional coprological examination, the majority of which are outdated yet highly valuable for studying digestive functions. In this regard, the present article aims to provide professionals in clinical analysis and related fields with a review, offering compiled and updated information for a more practical reference on functional coprological examination.
FUNCTIONAL COPROLOGICAL EXAMINATION
Pre-analytical phase
Patient preparation
For the performance of the functional coprological examination, a diet rich in substances that will facilitate diagnostic observation is recommended. The diet should include carbohydrates, fats, and proteins. Standardizing a test regimen that includes all digestible foods will facilitate the interpretation of the examination and allow for the comparison of the obtained results, enhancing the potential for investigating the functions of the gastrointestinal system.(2)
The classic regimens recommended by Schmidt-Strasburger for examining digestive functions do not always align with the customs and caloric needs of the inhabitants of each country, such as Brazil. However, the test regimen created by other gastroenterologists followed the same line of fundamental foods proposed by Schmidt-Strasburger.(1,4) Patients are advised to follow a normal diet for three days (72 hours) that includes meat, milk, potatoes, beans, and butter. The patient maintains their dietary habits while adding the necessary and fundamental foods for the examination. It is also advisable to recommend thorough chewing and to caution against the use of any medications such as antacids, digestive enzymes, antispasmodics, laxatives, and antidiarrheals. The use of carbonated and alcoholic beverages should be avoided.(1,2,4)
In infants and children up to 12 years of age, the criteria for the test regimen for digestive evaluation do not apply.(1)
It is important that the patient receives clear and detailed information about the test diet both verbally and in writing, along with recommendations regarding the importance of adhering to the diet accurately.
Stool collection
Collect the stool on the fourth day after the test regimen. If constipation occurs, extend the diet for one or two additional days, but never use laxatives, as they will interfere with the results and interpretation of the test.(1,2)
Collect all fecal material or approximately 50g of the middle portion of the stool in a clean, dry, wide-mouthed plastic container with a screw cap. Avoid contamination with urine, water, and soil. Do not collect stool from the toilet. For collecting stool samples from children who still use diapers, place a urine collection bag around the anal region. The stool must be fresh and free from preservatives. Send the fecal material to the laboratory immediately (within a maximum of 2 hours) to ensure the examination is conducted as quickly as possible, considering the potential for fermentation, putrefaction, and degeneration of the observed elements.
The instructions for stool sample collection should be provided to the patient both verbally and in writing or digitally.
Analytical phase
Macroscopic Examination and Physical Characteristics of Stool
Fecal samples must be examined macroscopically to determine the physical characteristics of the stool, such as consistency, appearance, shape, odor, color, viscosity, abnormal elements, and food residues.(3) The macroscopic examination also allows for the detection of adult worms and proglottids.
Weight: The weight of stool eliminated in 24 hours directly correlates with the quantity and quality of food consumed. The normal volume of stool ranges from 100g/day to 150g/day, potentially reaching up to 250g/day with a more abundant diet, depending on the amount of fiber ingested.(1,4)
Interpretation and clinical correlation: Certain pathological conditions can interfere with the volume of fecal mass. Thus, an increase in weight may be observed in cases of pancreatic insufficiency, inflammatory processes in the intestine, and intense intestinal fermentation. Conversely, in cases of constipation and low water intake, the volume is decreased.(1,4)
Consistency, appearance, and shape: Normal stool, considered solid, contains approximately 75% water; soft or pasty stool contains about 80% water; and diarrheal or liquid stool contains approximately 90% water. Normal stool is cylindrical, shaped by the anal sphincter.(1,4) It is recommended to evaluate the stool using the Bristol Stool Scale for consistency (Figure 1). This medical scale was developed at the University of Bristol, England, to classify human stool into seven types of shapes and consistencies.(3,5)
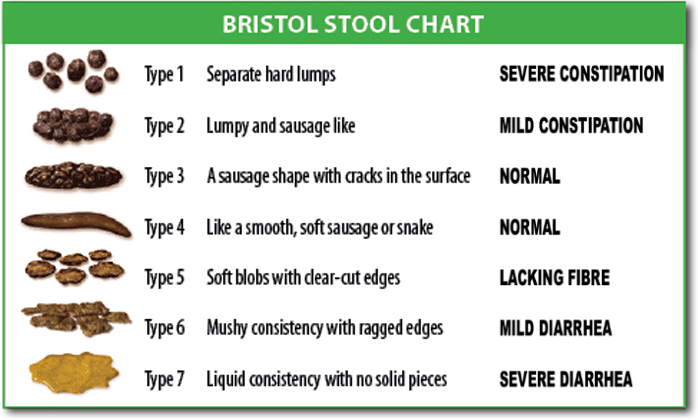
Figure 1
Bristol Stool Scale for stool consistency.
Source: https://pt.wikipedia.org/wiki/Escala_de_Bristol#/media/Ficheiro:BristolStoolChart.png
Color: Normal stool is brownish in color, which results from the presence of stercobilin, one of the pigments derived from bilirubin. Under different conditions, stool can appear greenish, yellowish, black (melena), red, or pale. The color may vary with the intake of foods such as vegetables, medications, and even underlying pathologies.(1,4)
Interpretation and clinical correlation: Green stool generally indicates excessive consumption of dark green vegetables, such as kale, broccoli, watercress, arugula, and spinach.(1) Black stool or melena may indicate digested blood from the upper parts of the digestive tract or the consumption of foods or iron supplements; red stool may indicate the presence of undigested blood or may occur after the intake of a large quantity of beetroot. Stool may be discolored due to the absence of stercobilin and pathological conditions.(1,4)
Odor: Normal stool has a characteristic fecal odor, sui generis or foul.
Interpretation and clinical correlation: A buttery or rancid odor in stool is associated with intestinal fermentation; a putrid odor indicates intestinal putrefaction. In colon and rectal carcinomas, the stool odor is putrid.(1,4)
Viscosity: Viscosity is low in normal stool. In intestinal putrefaction and colitis, stool is very viscous.(1,4)
Abnormal elements: Presence or absence of mucus, pus, and blood.
The presence of a small amount of mucus in the stool is normal. However, the presence of abundant mucus or bloody mucus is a sign of irritation or inflammation of the intestinal tract and should be investigated.(3,4) Visible blood may also indicate problems in the lower gastrointestinal tract, such as hemorrhoids or anal fissures.
Food residues: To disperse macroscopic debris, it is recommended to perform this analysis after diluting a portion of the fecal sample with water (10 mL), transferring the dilution onto a Petri dish, spreading it in a thin layer, and observing it against a black background. In stool, one may find seeds, beans, and corn kernels. In cases of pancreatic insufficiency or accelerated intestinal transit, fragments of carrot, apple, meat, or potato may be observed.(1,2,4)
Chemical Examination
Reaction and pH determination
Stool has a neutral,c weakly acidic, or weakly alkaline reaction, meaning that the pH of stool typically ranges between 6.8 and 7.2. In children, especially in infants, the pH may be between 5.0 and 6.0.(1,4)
The determination of reaction and pH is an important procedure that does not require the use of a pH meter. It is a colorimetric method, and pH indicator paper or pH indicator strips are commonly used for this determination in laboratories.(1,4)
Procedure: homogenize the stool using the tip of a glass rod or wooden stick, collecting samples from different points of the fecal mass, and apply a small portion to the pH indicator paper strip. The paper will change color, which should be compared to the corresponding color on the scale representing the pH of the stool.(1,4)
Fecal Dilution
In a plastic container or beaker, prepare a 10% stool dilution (10 g for 100 mL of water) by collecting samples from various parts of the fecal mass, gradually mixing in distilled or deionized water, and dissolving the stool with a glass rod until a well-homogenized suspension is obtained.(1,4)
Research on Reducing Substances
The presence of reducing substances in the stool may indicate a congenital deficiency of intestinal disaccharidases (lactase, maltase, sucrase) or a nonspecific injury to the mucosa. The malabsorption of different sugars caused by these enzymatic deficiencies leads to the emergence of reducing substances and a decrease in stool pH.(1,4) Intolerance to disaccharides, particularly lactose, is the most predominant carbohydrate intolerance in childhood.(6)
The colorimetric method using Benedict’s reagent is generally employed to detect the presence of reducing sugars. The test is essentially qualitative.(7)
Procedure: for the examination to be valid, the stool must be fresh (analyzed in the laboratory within a maximum of 1 hour).
In a test tube, add:
Benedict’s reagent ………………………………….2 mL
Diluted stool …………………………………………..5 drops.
Mix and heat over a Bunsen burner until boiling.
Result and interpretation: The change in color from blue to brick-red in the reagent indicates a positive reaction. Colorimetric variations may occur depending on the concentration ranges of sugars, ranging from intense blue to shades of green, yellow, and finally to brick-red.
Readings of (+), (++), (+++), (++++), correspond to the intensity of color, indicating the amount of reducing sugars in the fecal sample.
False results may occur in non-fresh fecal material due to the fermentation of sugars by intestinal bacteria.
Reference value: Non-reactive.
Research on Bile Pigments
Stool appears brownish due to the presence of stercobilin, which is the normal bile pigment. The presence of bile pigments is verified by the reaction of the sublimed compound.(1,2)
Procedure: In a test tube, add:
Diluted stool ……………………………………………………….5 mL
Saturated aqueous solution of mercuric chloride ……..5 mL
(33 g of mercuric chloride/500 mL of water)(2)
Shake and let it rest for a period of 5 minutes to 24 hours for reading.
Result and interpretation: A brick-red color of the liquid and sediment indicates the presence of stercobilin or stercobilinogen; a green color indicates the presence of bilirubin; no coloration indicates the absence of bile pigments.
Clinical Correlation: The absence of stercobilin may indicate biliary obstruction.(1,4)
Research on Albumin in stool
The sublimate reaction is also used to detect albumin in stool, employing the same reagent and procedure as described previously for bile pigments.(1,2)
Procedure: In a test tube, add:
Diluted stool ……………………………………………………….5 mL
Saturated aqueous solution of mercuric chloride ……..5 mL
(33 g of mercuric chloride/500 mL of water)(2)
Shake and let it rest for a period of 5 minutes to 24 hours for reading.
Result:
Negative Reaction: Sedimentation of fecal residues with turbid supernatant and no clumps.
Positive Reaction: Coagulation of proteins (flocculation) with a clear supernatant liquid.
Reference value: Absence.
Clinical correlation: Diagnosis of intestinal mucosal lesion and ulcerative colitis.(1)
Microscopic Examination
Stool Leukocyte Analysis
This test should be performed on fresh, recently collected stool samples and sent to the laboratory immediately or within 2 hours after collection.(2,4)
Direct fresh microscopic examination or staining with Lugol’s solution is used for leukocyte detection (Figure 2). The use of methylene blue stain facilitates easier visualization of polymorphonuclear leukocytes.(2,4)
Procedure:
Direct fresh examination and after staining: On a clean, dry slide, place a drop of diluted stool or a portion of stool from various points of the fecal sample, preferably if mucus is mixed with the stool; cover with a 22mm x 22mm coverslip and observe under a microscope at 400x magnification.
For the Lugol preparation, add a drop of Lugol’s solution and mix with the stool portion on the slide, then proceed as described above.
For the methylene blue preparation, mix the stool portion with the stain and proceed as previously described.
Result: Positive: presence of leukocytes or pyocytes (when degenerated), which should be quantified as rare, few, many, or numerous; Negative: absence of leukocytes in the fecal sample.
Note: For the detection of neutrophils and eosinophils, prepare a thin smear of diluted stool on a slide, stain with hematological stains, and examine under a microscope at 1000x magnification.
Interpretation: The leukocyte detection test in stool aims to verify the presence and quantity of leukocytes in the intestinal tract, which may indicate an inflammatory or infectious process. In intense infectious inflammatory processes, leukocytes are present in large quantities and appear clustered.(2,4)
Reference Value: Absent.
Clinical Correlation: A large number of leukocytes may be caused by ulcerative colitis, bacterial infection, cancer, tuberculosis, or mebiasis.(2,4)
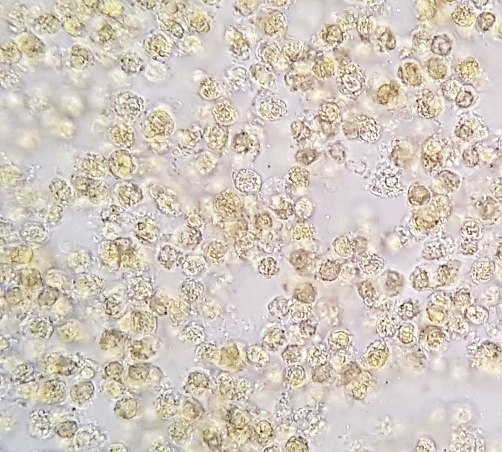
Figure 2
Leukocytes in fecal sediment stained with Lugol. 400x magnification.
Source: Courtesy of Lenilza Mattos Lima.
Food Residues and Elements of Intestinal Origin
Various elements can be found in stool, including food residues of animal and plant origin, as well as elements of intestinal origin. Thus, stool may contain residues of digested and undigested food, primarily of plant origin such as starch and cellulose; it may also contain epithelial cells and microorganisms such as yeast and bacteria.(1,2,8)
After stool dilution, prepare a fresh slide and another with Lugol’s solution, cover with a 22mm x 22mm coverslip, and examine under a microscope at 100x and 400x magnification.(1,2) It is recommended to analyze the fecal sediment after allowing the dilution to rest for approximately 30 minutes.
Result: For qualitative reporting, the element should be reported as present or absent; for quantitative reports, the structures observed under the microscope should be quantified as rare, few, many, or numerous.
Food Residues of Animal Origin
Muscle Fibers
Microscopic examination of fecal sediment may reveal well-digested and poorly digested muscle fibers, typically stained yellow or orange by bile. Well-digested muscle fibers appear oval, circular, or with rounded edges, lacking striations and angles (Figure 3). Poorly digested fibers appear as rectangular or elongated cylinders, with distinct longitudinal or transverse striations (Figure 3).
Interpretation: In normal digestion, well-digested muscle fibers may be found, while the presence of poorly digested muscle fibers in large quantities indicates accelerated intestinal transit or pancreatic insufficiency.(1,2)
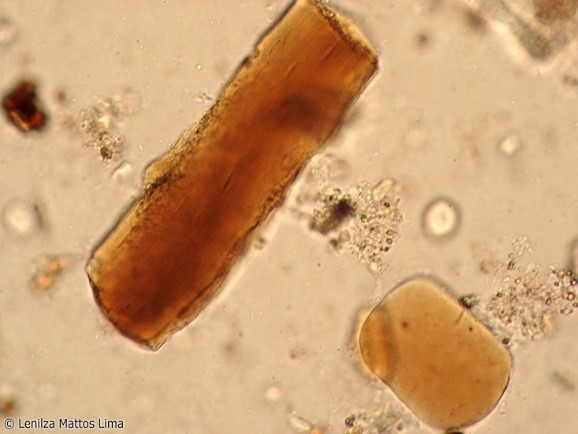
Figure 3
Muscle fibers in fecal sediment stained with Lugol: well-digested (rounded angle) and poorly digested (rectangular and striated).
Source: https://parasitologiaclinica.ufsc.br/
Food Residues of Plant Origin
Digestible Cellulose
Digestible cellulose constitutes the framework of feculent cells. Under microscopic examination, it appears isolated or in groups, oval, rounded, or polyhedral, with distinct contours and internal septa. It has a brownish coloration and may contain starch granules (included starch). This cellulose has semiological significance (Figure 4 A-B).
Interpretation: In normal stool, it appears in insignificant quantities, while its presence in large amounts may indicate accelerated intestinal transit.(2,4)
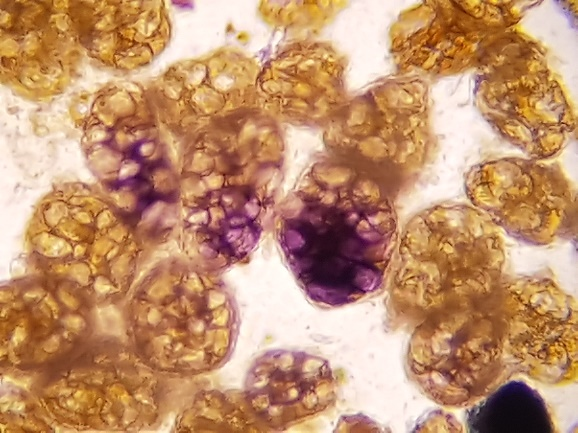
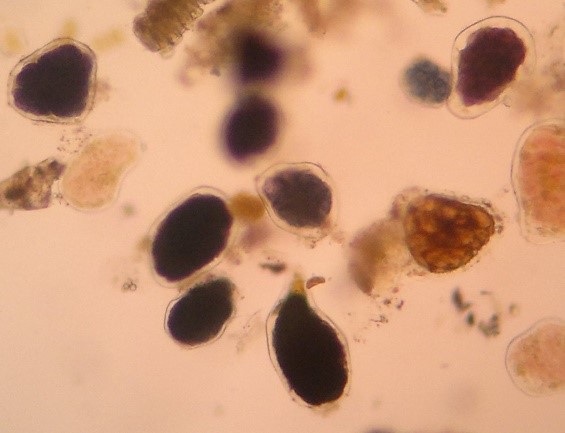
Figure 4 A and B
Digestible cellulose without starch and with included starch in fecal sediment stained with Lugol. 400x magnification.
Source: Courtesy of Lenilza Mattos Lima.
Undigestible Cellulose
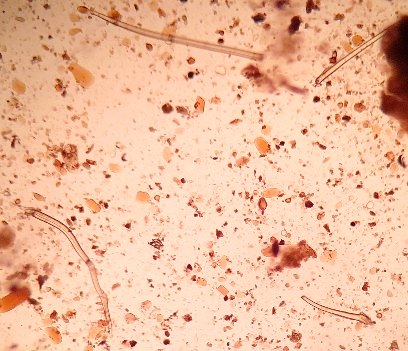
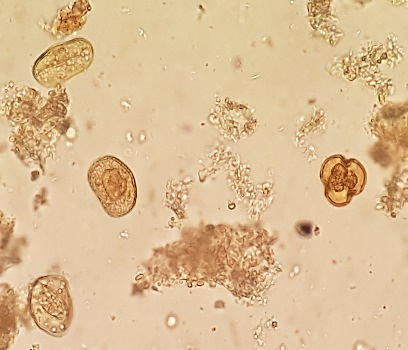
Undigestible cellulose is composed of plant fibers, cereal cuticles, vessels and plant trichomes, cellulose rings, palisade cells, pollen grains with variations in shape and size, spores, stomata, and other plant structures, which have no semiological significance.(2.8) Figure 5 (A, B, C, D, E) shows undigestible cellulose in fecal material.
 |
 |
 |
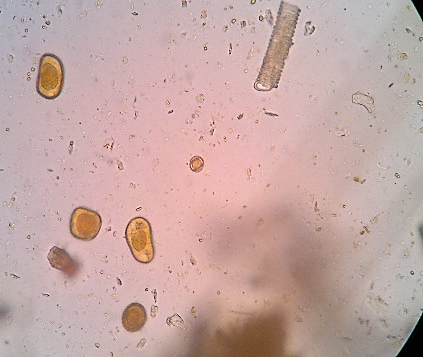 |
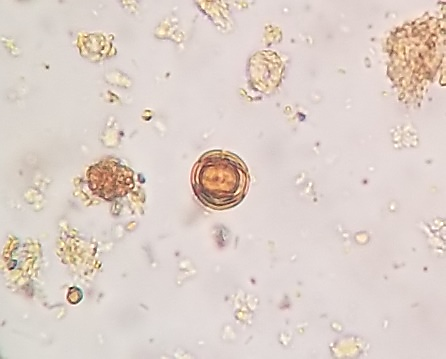
Figure 5 A, B, C, D, and E
Undigestible cellulose in fecal sediment stained with Lugol.
In A and B: plant trichomes (400x, 100x); in C and D: pollen grains (400x, 100x); in D, a spiraled plant vessel can be observed at the top; in E: pollen grain (400x).
Source: Courtesy of Lenilza Mattos Lima.
Starch
Starch is found in foods such as potatoes, rice, wheat, cassava, corn, oats, and fruits, which are part of the human diet. Under microscopic examination of fecal preparations on slides, starch appears in different forms: intracellular or included starch, raw starch, and amorphous starch. Intracellular starch appears within the cells of starchy foods, in the form of small whitish grains that are oval or round (Figure 4 A-B). Raw or undigested starch is found as oval or rounded grains, mostly organized in concentric layers (Figure 6). Amorphous starch generally appears as isolated or irregular bands and may be covered with iodophilic bacteria, with identification performed using Lugol’s solution. In preparations stained with Lugol, starch stains black, pink, or intense blue.(1,2,4)
Interpretation: A large amount of starch in the stool, particularly undigested starch, may indicate accelerated intestinal transit, excessive intake of starchy foods, poor mastication, or hydrocarbon fermentation in the intestines.(1,2)

Figure 6
Starch grains in Lugol-stained fecal sediment. 400x magnification.
Source: Courtesy of Lenilza Mattos Lima.
Iodophilic Flora
Normally found at the level of the cecum and right colon, it consists of bacteria that contain starch. In fecal preparations on slides stained with Lugol, they acquire a violet, blue, or black coloration (Figure 7 A-B).
Interpretation: In cases of accelerated intestinal transit or intestinal fermentation, iodophilic flora appears in large quantities. (1,4)
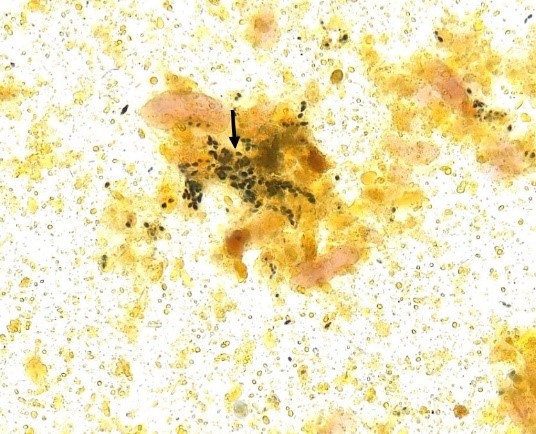
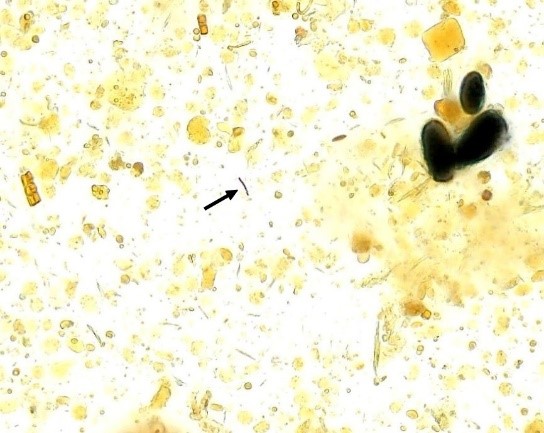
Figure 7 A and B
Iodophilic flora in fecal sediment stained with Lugol’s solution. 400x magnification.
Source: https://controllab.com/ensino/atlas/coprologia-funcional-ii/
A B
Elements of Intestinal Origin
The most common substances of intestinal origin are erythrocytes, leukocytes, and epithelial cells. Erythrocytes are observed with their known color, shape, and sizes, either isolated or agglutinated.(2) The presence of leukocytes in the intestinal tract is described above in “leukocyte detection in stool.” Epithelial cells are not found in normal stool, only if intestinal transit is accelerated.(2)
Other Elements
Other elements, such as fungi and crystals, described below, may also be found in stool samples.
Fungi in Stool
Yeasts and other fungal elements are common in stool. Fungi in stool may be transmitted through food and generally do not cause digestive symptoms or signs. They are recognized by their oval or spherical shape, brown coloration, and some by the presence of budding mycelium. Yeasts can produce pseudohyphae. The presence of yeasts from the genus Candida in stool may indicate intestinal candidiasis.(2) Figures 8 and 9 show the presence of fungi in stool.
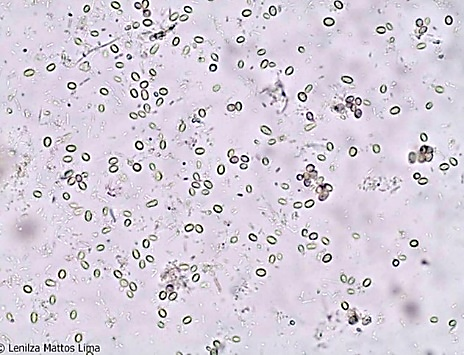
Figure 8
Fungi in fecal sediment. 400x magnification.
Source: https://parasitologiaclinica.ufsc.br/

Figure 9
Fungi in fecal sediment stained with Lugol’s solution. 400x magnification.
Source: Courtesy of Leticia Porto Coelho.
Crystals in Stool
Various crystals may appear in stool,(2,4) but those of greatest interest in digestive functions are:
- a) Calcium Oxalate Crystals: They appear in the form of envelopes. Interpretation: They arise after the ingestion of tomatoes, beans, green beans, and other vegetables. They are dissolved by hydrochloric acid in the stomach, and when found in large quantities in the stool, may indicate gastric insufficiency.
- b) Ammonium Magnesium Phosphate Crystals: They appear in the shape of coffin lids and in various sizes. Interpretation: They arise in alkaline stools with putrefactive dyspepsia. They may also appear during the collection of stool, contaminating it with urine.
- c) Fatty Acid Crystals: These appear as fine, long, and intercrossed needles, forming bundles.
- d) Charcot-Leyden Crystals: These have the shape of larger or smaller diamonds, elongated with pointed ends (Figure 10), and are derived from eosinophils.
Interpretation: They are associated with parasitic infections, allergies, and ulcerations. Charcot-Leyden crystals are observed when there is an inflammatory infiltrate with a predominance of eosinophils.
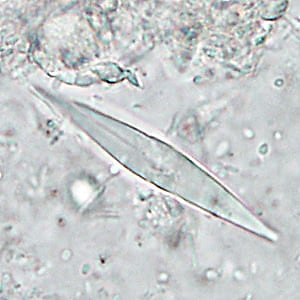
Figure 10
Charcot-Leyden crystal.
Source: https://www.cdc.gov/dpdx/artifacts/index.html
Bacterioscopic Examination
To verify the presence of Gram-positive and Gram-negative bacteria in Gram-stained fecal smears. The presence of other elements (cells, yeasts, and leukocytes) can also be observed.(4)
Fats in Stool
Fat digestion normally occurs in the small intestine, facilitated by the action of pancreatic enzymes, such as lipase. Normal stools may contain a small amount of fat in the form of neutral fats, fatty acids, and soaps. The presence of large amounts of fat excreted in the stool, defined as steatorrhea, may indicate intestinal malabsorption.(1,9,10)
Sudan III Staining
The detection of fecal fat is performed through microscopic examination with Sudan III dye (1% solution in 70% alcohol). This is a qualitative screening test that is easy to perform and shows good correlation with the measurement of fecal fat (described below) in the investigation of steatorrhea.(1-3) The Sudan III test has been reported to have a sensitivity of 77% and specificity of 98%.(3)
The patient should collect the stool without the use of laxatives and/or suppositories, and should not apply ointment to the anal region, castor oil, liquid vaseline, or mineral oil, as these can simulate neutral fats.
Procedure: On a clean, dry microscope slide, place one to two drops of diluted stool, add one to two drops of Sudan III solution, cover with a coverslip measuring 22mm x 22mm, and observe under a microscope at 400x magnification.
Result and interpretation: Neutral fats appear as droplets or globules, or in the form of red-stained lakes. The visualization of 60 or more droplets of neutral fat per field, stained red, indicates steatorrhea.(2)
Reading: The observation of 10 to 20 globules with a diameter of 10 μm or more is considered (+); 20 to 100 globules with a diameter of 10 to 50 μm is considered (++); and more than 100 globules of fat with large diameters are considered (+++).(3)
Reference Value: Absence/Rare
Clinical correlation: The diagnosis of steatorrhea may indicate pancreatic insufficiency (absence of lipase), cystic fibrosis, and parasitic infections, such as giardiasis and cryptosporidiosis.
Steatocrit (Micromethod by Phuapradit)
The steatocrit was developed by Phuapradit and colleagues (1981)(11) to assess the fat concentration in the stools of children. It is a semi-quantitative method based on microcentrifugation, which is easy to perform and low-cost, and it shows good correlation with fat balance in the evaluation of steatorrhea.(11,12) It has a sensitivity, specificity, and positive predictive value of 100%, 95%, and 90%, respectively.(13) The procedure described below by Mello and Silveira(12) is based on the original method by Phuapradit and colleagues.(11)
The patient should collect the stool without the use of laxatives.
Procedure:
- a) Use a glass rod to homogenize the stool to ensure an even distribution of the fecal mass;
- b) Separate a standard aliquot of stool (approximately 5 g);
- c) Mix this small amount of stool with 0.06 g of fine sand using a mortar;
- d) Add distilled water (4 volumes of the standard measurement) to dilute the stool, mixing again with the mortar, and homogenize for 1 minute with a vortex mixer;
- e) Aspirate this homogenized stool mixture into a heparin-free micro-hematocrit capillary tube;
- f) Centrifuge for 15 minutes in a micro-hematocrit centrifuge at 12,000 revolutions per minute. After centrifugation, the capillary tube is immediately placed in a vertical position, resulting in the formation of three layers: a lower solid layer (sand and solid residues); an intermediate liquid layer (water and soluble substances); and an upper layer that constitutes the fat in the stool.
Using a millimeter ruler with a precision of 0.025 cm and with the aid of a magnifying glass, measure the solid layer (S) and the fat layer (G).
Result: expressed as a percentage using the formula % steatocrit = G/(G+S) x 100
Reference values: Infants in the first week of life: up to 25%; from the 1st to the 4th week: up to 13%; from 1 to 3 months: up to 7%; over 3 months: up to 2%; adults: up to 2%.(12)
Clinical correlation: Diagnosis of fat malabsorption in children and adults, pancreatic insufficiency, and cystic fibrosis.
Additional tests
Additional tests include fecal occult blood testing, fecal fat quantification, fecal calprotectin determination, and measurement of alpha-1 antitrypsin in stool.
Fecal Occult Blood Test
Gastrointestinal bleeding may not be clinically apparent, and fecal occult blood testing assists physicians in diagnosing various clinical conditions by detecting the presence of small quantities of blood in the fecal sample.(14,15)
Several methods described in the literature are based on the detection of hemoglobin in feces, relying on the catalytic effects of the heme group, particularly the peroxidase activity of blood. Among these methods are the benzidine reaction, Meyer-Johannessen reaction, and guaiac reaction.(1,2) These colorimetric techniques require the patient to follow a specific diet for 3 to 4 days prior to the test. The patient should avoid consuming meats, chlorophyll-rich vegetables (such as spinach, lettuce, broccoli, radishes, turnips, beets, etc.), bananas, melons, pears, apples, as well as iron-based medications, corticosteroids, and aspirin.(1,15)
The immunochromatographic method is specific for the detection of human hemoglobin in stool. This method utilizes a combination of labeled monoclonal or polyclonal antibodies against human hemoglobin. The presence of hemoglobin from other animals does not interfere with the results, and there is no requirement for a specific diet prior to the examination.(14,15) The technique is performed according to the instructions provided in the package inserts of the test kits.(14)
Therefore, the immunochromatographic method for fecal occult blood testing can be conducted upon request for functional stool analysis.
Clinical Correlation: The fecal occult blood test is employed in screening for colorectal cancer and other gastrointestinal lesions, such as ulcers, diverticulitis, and esophageal varices.(14,15)
Fecal Fat Quantification
Fecal fat quantification for diagnosing suspected intestinal malabsorption typically involves a 72-hour stool collection, processed using the Van de Kamer titration method (2). Fecal fat excretion above 7 g/day supports the diagnosis of steatorrhea. It is essential for the patient to follow a balanced diet of 100 g of fat per day during the 3-day stool collection period.(1-3)
Clinical Correlation: The quantification or balance of fat in feces is useful in investigating malabsorption syndromes of pancreatic origin, cystic fibrosis, celiac disease, and Crohn’s disease.(2)
Calprotectin Determination in Stool Samples
Calprotectin is a protein found in the cytosol of neutrophils and macrophages, composed of two subunits, S100A8 and S100A9. It remains stable in stool for up to 7 days at room temperature and is evenly distributed throughout the stool sample. Calprotectin is classified as a damage-associated molecular pattern (DAMP) protein with antimicrobial protective properties. The extracellular release of calprotectin during periods of cellular stress or damage makes it an accurate marker of intestinal inflammation.(3,16)
In clinical laboratories, the enzyme-linked immunosorbent assay (ELISA) technique is employed to measure the concentration of fecal calprotectin. Several ELISA kits are commercially available.(16,17) Additionally, immunochromatographic tests have been developed for the diagnosis of fecal calprotectin.
Clinical Correlation: Fecal calprotectin serves as a biomarker for intestinal inflammation and is useful in diagnosing inflammatory bowel diseases, assessing the response to medical therapy, and predicting clinical relapse. This fecal protein is elevated in patients with ulcerative colitis, Crohn’s disease, cystic fibrosis, and colorectal cancer.(3,16,17)
Measurement of Alpha-1 Antitrypsin in Stool
Alpha-1 antitrypsin is a plasma protein resistant to degradation by digestive enzymes. The measurement of alpha-1 antitrypsin in stool is a test that analyzes protein loss through the digestive tract. The radial immunodiffusion method has been employed to measure fecal alpha-1 antitrypsin, as well as immunoturbidimetric methods.(18)
Clinical correlation: Elevated values are found in diseases that cause protein loss, such as inflammatory bowel disease, celiac disease, cow’s milk intolerance, gastric carcinoma, gastrointestinal lymphomas, Whipple’s disease, allergic gastroenteropathy, congenital hypogammaglobulinemia, and Menetrier’s syndrome.(18)
Post-Analytical Phase
The report of the functional coprological examination should provide information regarding the macroscopic examination of the stool and its physical characteristics, chemical tests, and microscopic analysis. Additionally, it is pertinent for the report to include information about the diet recommended by the laboratory.
Below is a proposed template for the report.
| Functional Coprological | ||
| Macroscopic Examination
Physical Characteristics of Stool |
Result | Reference value |
| Consistence
Shape Color Odor Viscosity |
||
| Blood
Mucus Pus Food Residues |
||
| Chemical Examination | ||
| pH
(Universal indicator paper) Reducing Substances (Benedict’s reagent) Stercobilin (Sublimate reaction) Bilirubin (Sublimate reaction) Albumin (Sublimate reaction) Occult Blood (Immunochromatographic method) |
||
| Microscopic Examination | ||
| Leukocytes
Erythrocytes Epithelial Cells Undigested Muscle Fibers Well-Digested Muscle Fibers Digestible Cellulose Non-Digestible Cellulose Raw Starch Amorphous Starch Starch Inclusion Fungi/Yeasts Crystals Iodophilic Flora Parasites Neutral Fats (Staining with Sudan III) Gram Staining |
||
Steatocrit
(Micromethod of Phuapradit)
FINAL CONSIDERATIONS
Coprological examinations are conducted to assess the functioning of the gastrointestinal system and the functional status of its organs. The results of the functional coprological examination will assist in the diagnosis of intestinal malabsorption syndromes, inflammatory bowel diseases, pancreatic disorders, and infectious and parasitic diseases. The examination also indicates pathologies associated with gastrointestinal bleeding. Early diagnosis facilitates prompt treatment and improves the prognosis of the disease.
Some physicians request both the stool parasitology test and the functional coprological examination simultaneously. However, it is important to note that when only the functional coprological examination is requested, attention should be given to the microscopic analysis of the fecal sediment, as the presence of parasites may be observed and must be reported in the test results.
REFERENCES
- Vallada EP. Manual de exames de fezes: coprologia e parasitologia. Rio de Janeiro: Atheneu;1998.
- Lima AO, Soares JB, Greco JB, Galizzi J, Cançado JR. Exame de fezes. In: Lima AO, Soares JB, Greco JB, Galizzi J, Cançado JR. Métodos de laboratório aplicados à clínica. 8ª ed. Rio de Janeiro: Guanabara Koogan; 2001. p.5.1- 5.49.
- Kasırga E. The importance of stool tests in diagnosis and follow-up of gastrointestinal disorders in children. Turk Pediatri Ars, 2019;54(3):141-8.
- Silveira Junior AO. O exame coprológico e as funções digestivas. São Paulo: Livraria Editora Santos;1988.
- Martinez AP, Azevedo GR. Tradução, adaptação cultural e validação da Bristol Stool Form Scale para a população brasileira. Rev. Latino-Am. Enfermagem, 2012; 20(3): [7 telas]. Available at: DOI: https://doi.org/10.1590/S0104-11692012000300021
- Carvalho LC, Silva SCM, Moraes JB, Pissolatto GG, Fernandes RB, Faria JP, et al. A intolerância a lactose e a alergia a proteína do leite de vaca (APLV): as principais considerações clínicas. Res Soc Dev, 2022;11(7):2-9. Available at: DOI: http://dx.doi.org/10.33448/rsd-v11i7.29651
- Universidade Federal da Paraíba. Laboratório Didático de Bioquímica. Teste de Benedict. 2017. Available at: http://plone.ufpb.br/ldb/contents/paginas/teste-de-benedict-1. Accessed: August 20, 2024.
- Carli GA. Artefatos confundíveis com os estágios de diagnóstico dos parasitos. In: Carli GA. Parasitologia Clínica: seleção de métodos e técnicas de laboratório para o diagnóstico das parasitoses humanas. 2a ed. Rio de Janeiro: Atheneu; 2011.p.139-151.
- Wikipedia. Esteatorreia. 2020. Available at: https://pt.wikipedia.org/wiki/Esteatorreia. Accessed: Access: August 20, 2024.
- Campagnaro ED, Jáuregui, Aparicio A, Lobo D. Valores normales de la prueba sudan III en niños sanos menores de un año de edad. Arch Venez Puer Ped, 2012;75(1):16-19.
- Phuapradit P, Narang A, Mendonça P, Harris DA,Baum JD. The Steatocrit: a simple method for estimating stool fat content in newborn infants. Arch Dis Child, 1981;56:725-7. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1627297/. Accessed: August 20, 2024.
- Mello ED, Silveira TR. Esteatócrito: um método semiquantitativo de avaliação de gordura fecal-padronização do teste. J Pediatr, 1995;71(5):273-278.
- Kamath MG, Pai CG, Kamath A, Kurien A. Comparing acid steatocrit and faecal elastase estimations for use in M-ANNHEIM staging for pancreatitis. World J Gastroenterol, 2017;23(12):2217-2222.
- Kroth K, Caumo KS, Lima LM. Saúde dos idosos: pesquisa de sangue oculto nas fezes. RBAC, 2023;55(4):276-281
- Honório JC, Tizzot MRP. Análise dos métodos de pesquisa de sangue oculto nas fezes. Cadernos da Escola de Saúde. Curitiba, 2010;3:01-11.
- Smith LA, Gaya RD. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J Gastroenterol, 2012;18(46):6782-6789.
- Badawy AM, Ali AAE, El Ghany AMA, El Halim EFA, Mohamed HI, Nouh MAE. Calprotectin as a fecal marker for diagnosis and follow-up in patients with ulcerative colitis. Menoufia Med J, 2014;27(1):35-43. Available at: https://www.menoufia-med-j.com/journal/vol27/iss1/6/. Accessed: August 27, 2024. DOI: https://doi.org/10.4103/1110-2098.132726
- Strygler B, Nicar MJ, Santangelo WC, Porter JL, Fordtran JS. α1-Antitrypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterol, 1990;99(5):1380-1387.
Correspondence
Lenilza Mattos Lima
E-mail: [email protected]
