Main classical staining methods in bacteriology: applications, techniques, principles, and limitations
Principais métodos clássicos de coloração em bacteriologia: aplicações, técnicas, fundamentos e limitações
Renata Garcia Costa1, Wagner Thadeu Cardoso Esteves2, Joseli Maria da Rocha Nogueira1
1 Fundação Oswaldo Cruz, LabMicro – Departamento de Ciências Biológicas – Rio de Janeiro, RJ, Brasil.
2 Fundação Oswaldo Cruz, Setor de Campylobacter – Laboratório de Zoonoses – IOC – Rio de Janeiro, RJ, Brasil.
Received on Sep 25, 2024
Approved on Oct 09, 2024
DOI: 10.21877/2448-3877.202400198.en
INTRODUCTION
In microbiology, staining methods can be employed to aid in the identification of microorganisms, often enabling a presumptive and early diagnosis in their classification. These methods are based on cytomorphotintorial characteristics—that is, cellular composition, morphology, and affinity for stains—where structures can be visualized and differentiated with the aid of a microscope.(1)
Bacteria, in particular, are naturally transparent and possess a refractive index similar to their surrounding medium, rendering them translucent when observed under a light microscope. Therefore, the use of stains is necessary to enhance the contrast between bacterial cells and the background, facilitating visualization.(2,3)
Some methods support the differentiation of microorganisms and bacterial structures based on structural and chemical characteristics, often proving essential for guiding clinical diagnosis as well as research activities associated with One Health and other fields. These standardized techniques not only facilitate rapid and accurate identification of pathogens in clinical samples but are also essential for studies exploring microbial morphology, distribution, and interactions.(4)
The staining processes used in bacteriology can be classified as simple and differential stains.(5)
The first category encompasses methodologies that employ a single stain as a contrast agent, such as methylene blue, crystal violet, and carbol-fuchsin, with the purpose of enhancing the visibility of cellular forms and arrangements. The use of a single stain can reveal internal structural differences depending on the bacterium, as exemplified by methylene blue, which can highlight volutin granules produced by Corynebacterium diphtheriae, allowing visualization of red-stained metachromatic granules against the blue-stained bacillary body.(6) Additionally, simple stains may also include specialized stains designed to highlight specific bacterial cell structures, such as spores, flagella, or capsules—aspects that will be addressed in greater detail throughout this article.(5,7)
Conversely, differential stains are more specialized techniques that employ multiple stains, mordants, and decolorizers to distinguish cellular structures based on their chemical properties, such as the Gram staining and Ziehl-Neelsen staining techniques, which are considered essential tools in the laboratory diagnosis of clinically significant microorganisms,(7-9) and are also applicable in One Health research.
Although numerous phenotypic and genotypic tests for microorganism identification are currently available, advancements in diagnostics do not replace classic methods, as these remain valuable, cost-effective tools that provide essential and foundational information for bacterial identification, and can, in certain cases, quickly substitute more complex examinations for diagnosis, as in cases of diphtheria, meningitis, tuberculosis, leprosy, and some sexually transmitted infections.(10-14)
OBJECTIVE
Given the importance of this subject, this article aims to conduct a narrative review illustrating the “state of the art” associated with the main staining techniques used in bacteriology, highlighting their practical applications and contributions to microorganism detection in various scientific and clinical contexts, as these subjects are considered foundational for understanding bacteriology practice and can provide pertinent information, particularly for students in the health field, serving as a comprehensive resource for academic study.
METHODOLOGY
According to Rother,(15) narrative review articles are broad publications suited for outlining and addressing the development or “state of the art” of a particular topic from a theoretical or contextual perspective. They do not require the disclosure of methodology for reference searches or criteria used in the evaluation and selection of works. They consist primarily of literature analysis published in books, printed and/or electronic journal articles, through the authors’ personal interpretation and critical analysis. Nevertheless, we chose to specify our selection criteria, which was based on research conducted in microbiology books available at the Department of Biological Sciences – ENSP/FIOCRUZ, the Manguinhos/FIOCRUZ Library Collection, and scientific articles accessed on the World Wide Web, using the specified descriptors.
STAINING METHODS USED IN BACTERIOLOGY
Gram Staining Technique
Gram staining is the most widely used differential technique in routine clinical bacteriology. Its purpose is to classify microorganisms according to their staining properties, size, shape, and cellular arrangement.(1) The Gram staining technique was developed by Danish physician Hans Christian Joachim Gram(2) in 1884. The method involves subjecting a heat-fixed bacterial smear to a successive series of stains, allowing the differentiation of microorganisms based on the chemical composition and integrity of their cell walls(2) into two major groups: Gram-positive and Gram-negative. The main component of the cell wall is peptidoglycan, also known as glycopeptide or murein, a biopolymer composed of alternating chains of N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc), which confer rigidity to the bacterial cell.(7)
The Gram-positive cell has a cell wall primarily composed of peptidoglycan and teichoic acid, whereas the Gram-negative cell is somewhat more complex, featuring a thin layer of peptidoglycan and an outer portion composed of lipopolysaccharides and lipoproteins (Figure 1 and Figure 2).
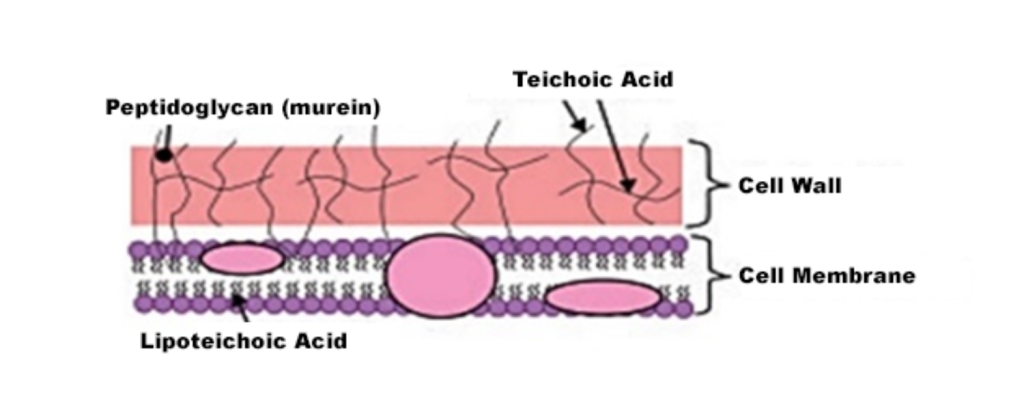 Figure 1
Figure 1
Basic structure of the Gram-positive cell wall(5)
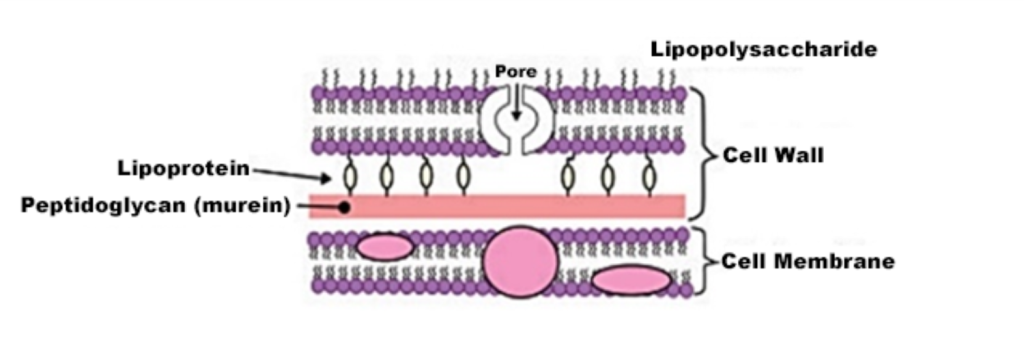 Figure 2
Figure 2
Basic structure of the Gram-negative cell wall(5)
In the classic Gram staining method, a thin, homogeneous, and dry smear from a bacterial culture or clinical material is treated with a primary stain (crystal violet with phenol) for 1 minute, which penetrates the cell wall and protoplasm. Following this, Lugol’s solution (an iodine-iodide solution) is applied for 1 minute as a mordant, forming an insoluble stain-iodine complex in water, referred to as iodine-pararosaniline. Up to this stage, both Gram-positive and Gram-negative bacteria absorb the primary stain and fixative identically, initially acquiring a violet color. Subsequently, an organic solvent (alcohol-acetone) is applied for 30 seconds as a decolorizer. After this step, the slide is rinsed with water (a mandatory step) to remove any residual stain, and then it is covered with a 1/10 diluted Ziehl fuchsin (secondary stain) for 30 seconds, acting as a counterstain.(2) After the entire process, the slide should be dried and examined under a light microscope with a 100X oil immersion objective.(7)
It is important to note that the decolorizing solution acts differently depending on the structural characteristics and varying degrees of permeability of the cell wall. In Gram-positive bacteria, this solvent promotes the contraction of the peptidoglycan pores, retaining the primary stain and maintaining a purple color (Figure 3a). However, in Gram-negative bacteria, the solvent dissolves the lipid portion (lipopolysaccharides and lipoproteins) of the outer membrane, removing the crystal violet-iodine complex and adopting the color of the secondary stain (fuchsin or safranin), thus revealing a red color (Figure 3b).(9,7) The overall difference in observed tones arises from differences in the physical and chemical properties of bacterial cell walls, such as composition, thickness, density, porosity, and integrity.(7,16,17)
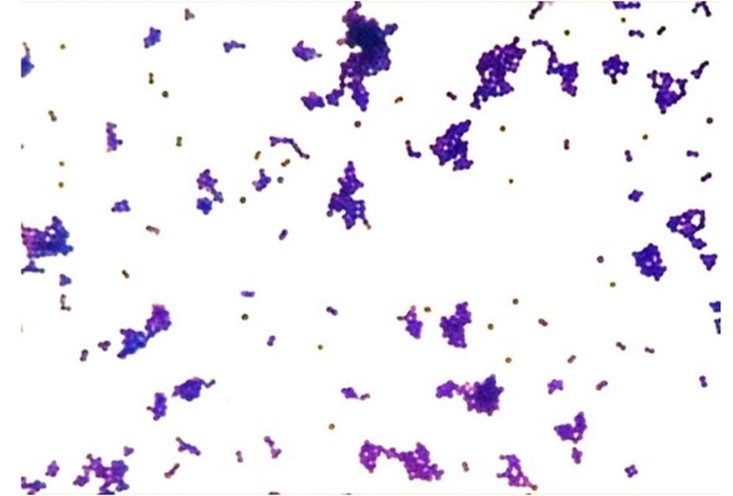
Figure 3a
Staining characteristic of Gram-positive (DCB, FIOCRUZ, 2024)
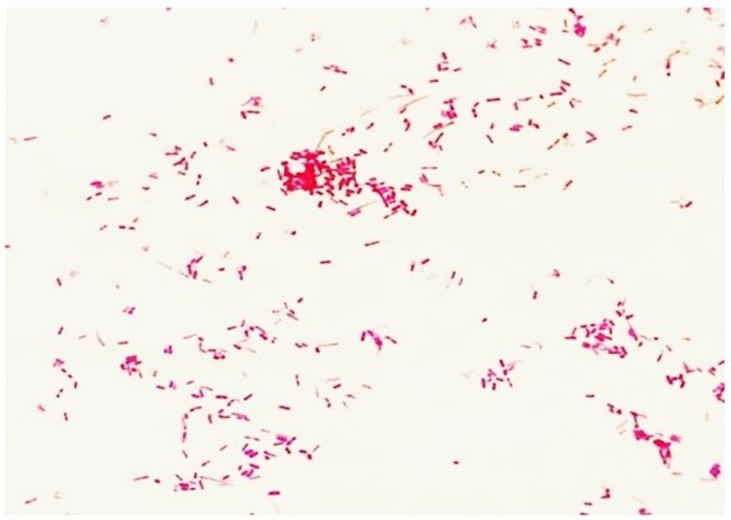 Figure 3b
Figure 3b
Staining characteristic of Gram-negative (DCB, FIOCRUZ, 2024)
In the literature, some studies suggest alternative methods, indicating modifications and adaptations to the technique. Some variations involve changes in stain concentrations, reagent substitutions, and adjustments in stain exposure times. This was initially based on the work of Lellis and colleagues (2019),(18) who highlighted the potential toxicity, carcinogenicity, and environmental impact of stains and the risks to human health due to improper disposal after laboratory use.(19)
In laboratory practice, Reine and colleagues(19) supported replacing the classic Gram and Wirtz-Conklin methods (on slide surfaces) with immersion staining, demonstrating its applicability for staining many slides simultaneously without compromising result reliability, avoiding solution saturation after multiple slide passes, reducing stain waste, and consequently lowering costs for technique application.
Currently, replacing crystal violet with methyl violet is recommended, based on its chemical composition that acts as a fixative, eliminating the need for flame fixing the smear—a practice now obsolete and contraindicated. Another adjustment is the substitution of the alcohol-acetone solution used by Hurk with 99.5° ethyl alcohol, considered safer and less toxic for the handler, preventing over-decolorization of the slide and making the technique more reproducible. However, the most significant modification was the replacement of phenol-fuchsin as the secondary stain with safranin. This change was based on studies regarding color spectrum, showing that safranin provides greater distinction and clarity between Gram-negative and Gram-positive bacteria, as it contrasts more distinctly with the primary stain compared to fuchsin, resulting in Gram-negative bacteria appearing light red and Gram-positive bacteria violet.(7,20)
GRAM STAINING TECHNIQUE
| CLASSIC METHOD | MODIFIED METHOD | |
| SMEAR PREPARATION | Prepare a thin and homogeneous smear on a previously cleaned and degreased slide; allow it to air dry, and then fix it by passing it three times over the flame of a Bunsen burner. | Prepare a thin and homogeneous smear on a previously cleaned and degreased slide, and allow it to air dry. In the modified method, fixation does not occur through heat but rather through the use of methyl violet, which acts as a chemical fixative. |
| SLIDE STAINING PROCEDURE | Cover the slide with crystal violet and let it sit for 1 minute.
Drain the stain and rinse with a gentle stream of running water (this step is optional but enhances visualization).
Cover the slide with Lugol’s solution and allow it to act for approximately 1 minute.
Remove excess stain and rinse with a gentle stream of running water (this step is also optional).
Apply alcohol-acetone for decolorization, allowing it to act for approximately 30 seconds.
Rinse with a gentle stream of water (mandatory step).
Cover the slide with diluted Ziehl-Neelsen fuchsin stain (1/10 dilution) and let it act for approximately 30 seconds.
Rinse with a gentle stream of water (mandatory step). |
Cover the smear with methyl violet and let it sit for approximately 15 seconds.
Add an equal amount of water over the slide covered with methyl violet and allow it to act for an additional 45 seconds.
Drain the stain and rinse with a gentle stream of running water.
Cover the slide with diluted Lugol’s solution (1/20) and let it act for approximately 1 minute.
Drain the Lugol’s solution and rinse with a gentle stream of running water.
Apply ethyl alcohol (99.5% GL) over the slide for decolorization, continuing until no more stain is released.
Rinse with a gentle stream of running water.
Cover the slide with safranin and let it act for approximately 30 seconds.
Rinse with a gentle stream of running water. |
| SLIDE OBSERVATION | Allow the slide to air dry naturally. Then, place it under the microscope and, using immersion oil, observe with the immersion objective(100X). | |
Source: Nogueira and Souza(5); Brasil(7)
MAIN MODIFICATIONS IN THE GRAM METHOD (7,20)
| CLASSIC METHOD | MODIFIED METHOD | JUSTIFICATION |
| Crystal violet | Methyl violet | Methyl violet has fixative properties, eliminating the need for heat fixation with a Bunsen burner, which can cause abrupt dehydration of cellular components |
| Lugol | Step removed | ——– |
| Alcohol-acetone | 99.5°GL ethyl alcohol | The 99.5°GL ethyl alcohol solution has lower toxicity potential and reduces hyper-decolorization effects on the slide |
| Phenol fuchsin | Safranin | In the color spectrum, safranin is further from the primary stain compared to fuchsin, allowing for greater differentiation and clarity between Gram-positive and Gram-negative bacteria. |
Source: Brasil(7); Pinto and Ribeiro(20)
It is important to note that certain bacterial groups do not stain using the Gram method, as they possess specific characteristics that require specialized techniques. For example, spirilla have an exceedingly thin cell body, making visualization difficult with classic methods; and the genus Mycobacterium, due to its unique cell wall composition,(3) requires a specialized staining approach. Additionally, bacteria that lack a cell wall, such as mycoplasmas, should also be considered. These bacteria measure only about 0.2 µm and can be observed only through electron microscopy or fluorescence microscopy.
ZIEHL-NEELSEN STAINING TECHNIQUE
The Ziehl-Neelsen technique is an essential methodology and remains one of the most commonly used methods in contemporary clinical bacteriology. Developed by bacteriologist Franz Ziehl and later modified by German pathologist Friedrich Carl Adolf Neelsen in the late 19th century, this method emerged from efforts to visualize Mycobacterium tuberculosis, the causative agent of tuberculosis, which could not be effectively stained by traditional methods due to its unique and distinctive cell wall.(3)
In addition to enabling the staining of the Mycobacterium genus, this technique serves as a differential method for identifying all acid-fast bacilli (AFB), including the genus Nocardia. The acid-fast characteristic of these bacteria is attributed to the high content of structural lipids in their cell wall, which promotes a hydrophobic condition that interferes with the permeability and action of aqueous stains, thereby conferring resistance to acid-alcohol reagents.(12,21)
Among the primary bacterial groups detected in clinical laboratories using this technique are the M. tuberculosis complex and Mycobacterium leprae, recognized by the World Health Organization (WHO) as agents of significant public health concern for clinical medicine.(11-13,22)
The M. tuberculosis complex currently comprises 11 distinct species associated with both human and animal tuberculosis. Among them, M. tuberculosis stands out as the primary cause of tuberculosis in humans due to its high level of adaptation, making it the most significant species in the complex from a public health perspective.(23) Tuberculosis is a transmissible infectious disease that primarily affects the lungs (pulmonary form) but can also involve other organs and/or systems.(22) Thus, the Ziehl-Neelsen staining technique is employed to detect AFB in a variety of clinical specimens, including urine, cerebrospinal fluid, biopsy samples, and pulmonary specimens such as bronchial lavage, gastric lavage, and laryngeal swabs. However, sputum smear microscopy is recognized by the Ministry of Health as the most critical method for both diagnosing and monitoring the treatment of pulmonary tuberculosis in the country.(12,22)
Due to its distinctive cell wall, which slows nutrient absorption, M. tuberculosis may take over a month to form visible colonies on isolation media.(9) In such cases, the detection of AFB in clinical specimens may serve as the first evidence of disease, enabling early diagnosis and providing a basis for the initiation of therapeutic treatment, while awaiting laboratory confirmation of a positive culture.(22)
Conversely, M. leprae is the etiological agent of leprosy, a disease characterized by peripheral nerve impairment and the formation of lesions and spots on skin areas, with altered thermal (heat and cold), motor, and/or anatomical sensitivity.(11,13,24)
The diagnosis of leprosy is primarily based on clinical and epidemiological criteria, as M. leprae is an unculturable microorganism in vitro. In this context, bacilloscopy remains among the essential primary tests for detecting bacteria in affected areas.(11)
The Ziehl-Neelsen technique is an easy-to-perform, rapid, and cost-effective method that detects the presence of AFB.(12) When properly executed, from sample collection and processing to bacilloscopy, it provides diagnostic efficacy in 80% of cases.(22)
In laboratory routine, M. tuberculosis detection is usually performed with spontaneous sputum, using the purulent portion, which is placed and spread directly onto the slide (direct bacilloscopy). Purulent secretions and pus, due to their physical characteristics, can also be spread directly onto the slide. However, to increase bacilloscopy sensitivity, all clinical samples, whenever possible and necessary, may undergo treatment before smear preparation. This treatment can include crushing, digestion with chemical agents such as sodium hydroxide (NaOH) or NaCl, concentration, sedimentation, flotation, or filtration.(12) The Ministry of Health recommends specific methodologies for sputum decontamination and treatment prior to bacilloscopy, including the modified Petroff method, the N-acetyl-L-cysteine-sodium hydroxide (NALC-NaOH) method, the swab-Ogawa-Kudoh method, and the oxalic acid method.(12)
Although M. tuberculosis is a highly hazardous microorganism transmitted through airborne transmission (Class III), the processing of clinical material for bacilloscopy follows Biosafety Level 2 (BSL-2) recommendations, with emphasis on careful handling of biological material during smear preparation.(12) According to the Laboratory Diagnosis Manual for Tuberculosis,(12)clinical material should be deposited in fluid form on the slide using sterile wooden sticks, Pasteur pipette, and/or platinum loop, as prescribed by preparation methods. Heating the slide during smear preparation is not recommended, not only due to the risk of aerosol formation in the laboratory environment but also to prevent granular precipitates that impair staining and bacillus detection. In this case, smear fixation should only be performed once the samples are completely dry.(12)
In the Ziehl-Neelsen staining technique, Ziehl’s fuchsin solution (carbol-fuchsin) is used as the primary stain (which is 10 times more concentrated than the fuchsin used in the Gram method). At this stage, heat treatment is necessary to facilitate dye penetration and binding within the mycobacterial cell wall. Therefore, after covering the slide with Ziehl’s fuchsin, it should be left to act for 5 to 10 minutes, warming it gently (avoiding boiling) until vapors form. Subsequently, the slide is briefly rinsed and decolorized with a 1% hydrochloric acid-alcohol solution. The slide is then counterstained with methylene blue for approximately 30 seconds, followed by rinsing, drying, and examination under oil immersion microscopy (100x).(12)
The distinctive characteristic of the mycobacterial cell wall lies in its peptidoglycan layer composition, consisting of N-glycolylmuramic acid and mycolic acids, which are long-chain fatty acids (lipids) that form a barrier and impede dye penetration. The lipids strongly bound to the mycolic acid structure promote hydrophobicity, preventing the penetration of aqueous dyes, mordants, and differentiating agents, a characteristic not present in other bacterial genera. The principle of the Ziehl-Neelsen technique is based on the fact that heating causes dilation, enabling carbol-fuchsin to penetrate the cell and remain trapped upon cooling, persisting even after decolorization with an acid-alcohol agent.(12)
During the staining process, Ziehl’s phenicated fuchsin (carbol-fuchsin) stains all cellular elements red. However, following acid-alcohol decolorization, only acid-fast bacilli (AFB) retain this coloration and become distinguishable from other microorganisms lacking this property. The decolorized cellular components subsequently absorb the background stain, methylene blue (counterstain), as illustrated in Figure 4.(5,12)
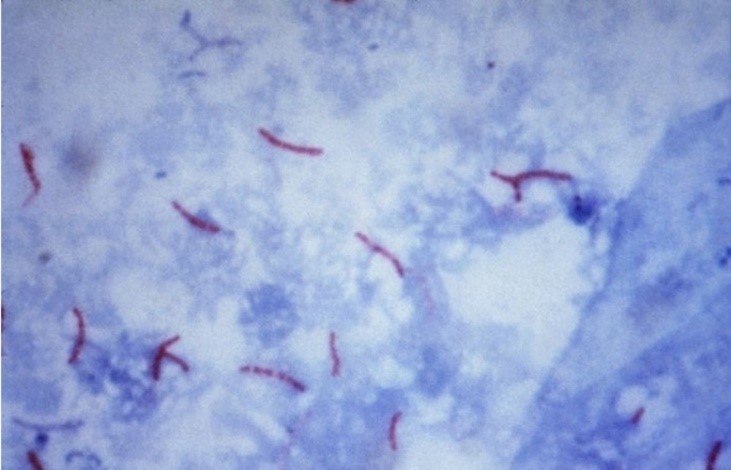
Figure 4
Mycobacterium tuberculosis stained by the Ziehl-Neelsen technique.(12)
According to the Ministry of Health,(12) the reading and interpretation of bacilloscopy results follow the criteria below:
Reading and interpretation of bacilloscopy results from sputum samples:(12)
| READING | RESULT |
| No AFB detected in 100 observed fields | NEGATIVE |
| 1 to 9 AFB in 100 observed fields | Report the quantity of bacilli found |
| 10 to 99 AFB in 100 observed fields | POSITIVE + |
| 1 to 10 AFB in 50 observed fields | POSITIVE ++ |
| More than 10 AFB per field, on average, in 20 observed fields | POSITIVE +++ |
Reading and interpreting the results of bacilloscopy of sputum samples.
Reading and interpreting the results of bacilloscopy of other clinical samples:(12)
| READING | RESULT |
| No AFB detected in 100 observed fields | NEGATIVE |
| No AFB detected in 100 observed fields | POSITIVE |
FONTANA-TRIBONDEAU METHOD
This method was devised by Alfonso Fontana and Louis Tribondeau(25) in 1920. While it is considered a laboratory staining practice, this characterization is somewhat misleading. It is, in fact, a silver impregnation technique employed for visualizing extremely thin spiral bacteria that do not stain adequately with the conventional Gram staining method, such as treponemes and leptospires. In this technique, spirochetes are treated with ammoniacal silver, allowing them to stand out against a light background. As a result, they become visible in dark brown or black against a yellow-brown or light brown background.(5,9,25)
The methodology entails depositing several drops of Ruge’s solution (glacial acetic acid, 40% formalin, and distilled water) onto the dry smear and allowing it to act for 30 seconds (repeated three times). This step serves to fix the smear onto the slide. This is crucial because, for these specimens, heat fixation, as performed in other methodologies, is not advisable; heating may adversely affect the morphology of the spirochetes.(5,9,25)
Following this step, the smear is covered with the mordant solution (composed of tannic acid and phenol) and subjected to heating until vapors are emitted for 30 seconds. Following this step, the smear is covered with the mordant solution (composed of tannic acid and phenol) and subjected to heating until vapors are emitted for 30 seconds. As illustrated in Figure 5, the silver is then reduced to metallic silver, depositing onto the spirochetes, which can be observed under a microscope with a brown coloration.(5,9)
One of the primary applications of this technique is its significance for the diagnosis of syphilis. From clinical material, it is possible to achieve direct visualization of Treponema pallidum, which cannot be cultured in vitro.(26)
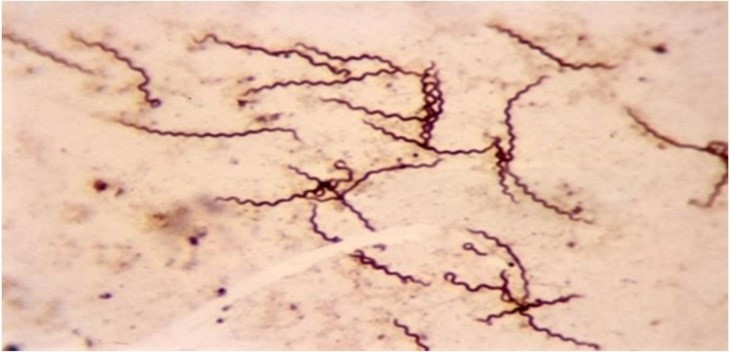
Figure 5
Treponema pallidum stained using the Fontana-Tribondeau method.(27)
ALBERT-LAYBOURN STAINING
This staining method was first proposed in 1920 by Henry Albert and later modified by Ross Laybourn(28) in 1924. This technique has been employed for detecting corynebacteria in the presumptive diagnosis of diphtheria and is based on the presence of cytoplasmic granules located at the poles of these bacteria, known as metachromatic granules or Babes-Ernst granules. These structures are composed of polyphosphates that accumulate within the cell when nutrients other than phosphate become scarce, particularly when sulfate is in limited supply. Under these conditions, nucleic acid synthesis is halted, and volutin accumulates in anticipation of its use for this synthesis. This polyphosphate-rich material stains intensely with strong Lugol’s solution, giving the impression of being larger than the bacillary body itself and acquiring a brown hue, which can be observed in contrast to the bacillary body that stains blue-green with Laybourn’s solution.(5)
The Albert-Laybourn staining method is considered the “gold standard” for screening Corynebacterium diphtheriae from direct examinations of nasal and oropharyngeal smears. This microorganism, responsible for a transmissible infectious condition, can progress to the formation of typical pseudomembranous plaques that lodge in the tonsils, larynx, pharynx, nose, and even in the conjunctiva and skin.(9,21,24) Although it is preventable through immunization and currently treated with antitoxin therapy, rapid diagnosis is a critical factor for a favorable outcome when an infection occurs.
Diagnosis of the disease is based on the assessment of clinical symptoms, which, when combined with optical microscopy as a complementary test, may reveal the presence of metachromatic bacilli in the collected clinical material, presumptively indicating diphtheria. However, the WHO recommends that the collection of clinical material and the performance of bacterial culture be conducted simultaneously to isolate Corynebacterium sp. in specific media for confirmation of the diagnosis.(21,24)
The Albert-Laybourn method involves covering the smear with Albert-Laybourn solution (composed of toluidine blue, malachite green, glacial acetic acid, 95% ethanol, and distilled water) for 3 to 5 minutes. The solution is then drained without washing, and the smear is covered with strong Lugol’s solution (comprising metallic iodine, potassium iodide, and distilled water), allowing it to act for approximately 2 minutes. After this process, the smear is washed, dried, and examined under a microscope using an immersion objective.(5) The metachromatic granules will appear brown, as shown in Figure 6.
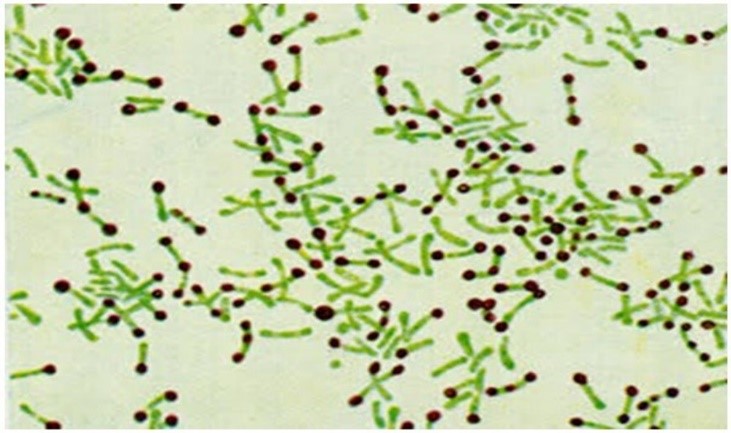
Figure 6
Metachromatic granules stained using the Albert-Laybourn method. (9)
SPORE STAINING
Certain bacterial groups, such as the Bacillus and Clostridium genera, are capable of forming resistant structures known as endospores. This phenomenon typically occurs when microorganisms encounter unfavorable conditions, such as nutrient scarcity, inadequate humidity, and improper temperature. Under these conditions, microorganisms enter a sporulation phase, producing within themselves a resistant spherical or ovoid structure, characterized by high calcium content associated with dipicolinic acid, which is linked to dehydration and enhanced resistance, including thermal resistance.(5,7) Each cell forms a single spore, which is released into the external environment after cell death. These structures can remain in a dormant or resting state in the environment for years until they encounter an optimal condition to become viable again.(16)
One of the primary functions of endospores is their protection against physical and chemical agents used in sterilization/disinfection processes, as they can only be destroyed by autoclaving at 120°C for 30 minutes.(5,7)
Despite the importance of their detection, visualizing spores under a light microscope can be challenging due to the refractile nature of these structures, which makes them resistant to various stains that cannot penetrate the endospore wall. In common staining procedures, the bacterial body is stained, while the endospore remains colorless.(7)
The Schaeffer-Fulton stain, developed by A.B. Schaeffer and M.D. Fulton(29) in 1933, is a notable spore staining technique for staining bacterial endospores associated with clinically significant human infections, such as tetanus and botulism, which are of great relevance to Public Health.(24) Another bacteriological staining method for spore detection is the Wirtz-Conklin stain,(30) described in 1837, which enables the visualization of these structures under a microscope. Currently, this methodology remains the standard for examining environmental and clinical samples, specifically for diagnosing diseases such as anthrax.(5)
To perform spore staining, a pure culture of the suspected strain is required. It is recommended to subject this culture to extreme temperatures for 24 hours to stimulate sporulation. For this purpose, the culture can be placed in an oven at 44°C or in a refrigerator (8°C) for 24 to 48 hours. It is important to note that if these temperatures are maintained for an extended period, only exospores will be observed, as all endospores will have exited the bacillus. At the end of this stage, a few drops of sterile saline solution are added to the slide and mixed with the prepared culture.(7)
The principle of both techniques is to use malachite green as the primary stain and safranin as the counterstain. In the Wirtz-Conklin technique, a homogeneous, thin, and fixed smear is first prepared and then covered with malachite green stain. In a beaker, water is heated until it begins to emit vapor, and the slide is placed above it, maintaining the stain heated for 5 minutes. Alternatively, the slide can be brought near a gentle flame until vapor is released, without allowing the stain to boil. This heating step is repeated every 1 to 2 minutes, for 3 to 4 cycles. Heat assists the stain’s penetration through the thick layer formed by the cortex. After this process, the slide is gently rinsed to remove excess stain, avoiding thermal shock and potential slide breakage. Safranin is then added to the smear as a counterstain, allowed to act for 30 seconds.
In the Schaeffer-Fulton method, the procedure is similar, respecting the time and using the same stains; however, it does not include a heating step. In both techniques, endospores appear green within red or pink cells when the smear is properly prepared. As shown in Figure 7, this allows the endospores to be visualized. Due to their high refractivity, endospores can be detected under a light microscope when unstained, but they cannot be distinguished from storage inclusions without specific staining.(5,7)
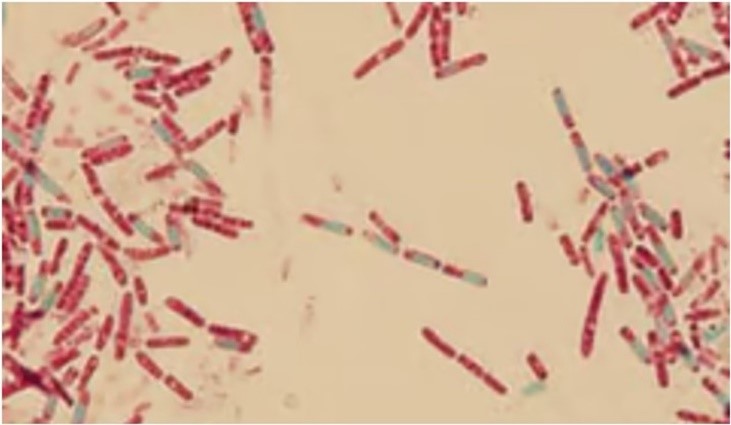
Figure 7
Spore staining using the Wirtz-Conklin technique(27,30)
Other, less common spore staining techniques are described in the literature, such as the Moeller stain, the Dorner stain, and the modified Dorner stain.(31)
The Moeller technique involves using chromic acid and fuchsin as the initial stain, with methylene blue as a counterstain. In this method, spores will appear red against a blue background. This technique involves covering a pre-prepared smear with alcohol, which is then flamed until it extinguishes naturally. Subsequently, the slide is covered with 5% chromic acid, left to act for 5 minutes. After sensitization, the slide is washed and phenol-fuchsin solution is applied for 10 minutes, with heating to the emission of vapor. The decolorization step follows, initially using either 5% sulfuric acid or 10% nitric acid, then finishing with absolute alcohol. The smear is then washed, counterstained with 1% methylene blue for 3 minutes, followed by washing and drying of the slide. Upon immersion microscopy, spores are observed as red, with the bacterial body stained blue.(31)
In the Dorner staining method for spores, fuchsin and nigrosin are used to sensitize and stain the spore. Nigrosin functions to extract all stain from cellular structures, except the spores, which take on a distinct (red) color compared to the bacterial body. In the Dorner technique, a concentrated suspension of sporulated microorganisms is prepared in distilled water, and an equal volume of filtered Kinyoun fuchsin (phenol-fuchsin) is then added. The tube is then placed in a boiling water bath for 5 to 10 minutes. On a clean slide, a drop of the suspension is mixed with a drop of 10% aqueous nigrosin solution, which has been boiled and filtered. This is spread and quickly dried with mild heat. Examination is done with a 100X oil immersion objective. In this technique, spores appear red, and the bacterial cells are nearly colorless against a dark gray background.(31)
In the Dorner modified technique, a smear of a fixed sporulated microorganism suspension is prepared, then covered with a strip of filter paper and phenol-fuchsin is added. The slide is then heated for 5 to 7 minutes until vapor is released. Afterward, the paper is removed, and the slide is washed and dried with absorbent paper. The smear is then covered with a thin layer of 10% nigrosin solution, spread with the help of a second slide. Under microscopic observation, the spores can be visualized with the same color as noted in the previous technique.(31)
Spore staining can be applied across various sectors. These include clinical, food industry, and environmental fields.(7,32-34)
In clinical settings, spore detection is crucial for identifying Clostridium tetani, the causative agent of tetanus.(7) This disease is caused by toxins released by the bacterium, which can form spherical terminal endospores with a characteristic “drumstick” appearance, easily observed through staining techniques.(24)
In the context of One Health, controlling endospores in food production poses a major challenge, as these structures are highly resistant and pose risks related to food spoilage and foodborne diseases, such as botulism.(24) Botulism is a rare bacterial disease caused by Clostridium botulinum and is often associated with the consumption of contaminated foods, particularly canned foods and those lacking proper preservation.(24,32) In quality control processes during food production, staining techniques are essential, as they allow for the rapid detection and quantification of spores.(33) Another application involves the examination of bacterial endospores in environmental samples, particularly in studies on the monitoring and efficiency of Water Treatment Plants (WTP), including raw water analysis at the intake and effluent from filtration units at the Wastewater Treatment Plant (WWTP), as stipulated by GM/MS Ordinance No. 888, dated May 4,2021.(34)
Still within the scope of bacterial staining techniques, it is important to highlight simpler methodologies that, although seldom used in routine laboratory procedures, hold significant value in research activities involving the study of specific bacterial cellular structures. These include capsule staining (negative staining or India ink, India ink staining with diluted fuchsin, Hiss staining) and flagella staining.(5,7)
NEGATIVE STAINING
The negative stain is so named because it stains the background rather than the bacterial body itself. It can be performed using a single stain (simple staining)(5), as in the case of India ink for capsule observation, or combined with other stains, such as in the Gins method.(35)
This technique is widely used to visualize capsules, which are structures present in certain microorganisms that, among many functions, protect the cell against phagocytosis by polymorphonuclear leukocytes and contribute to bacterial adhesion to cells and surfaces. This adhesion is essential for many organisms to establish infections in suitable hosts or to maintain the bacterial cell on a specific environmental surface.(35) The capsular material is typically composed of polysaccharides and phosphates with antigenic properties, which are often detected through serological tests. Among capsule-producing pathogenic bacteria, we can cite Streptococcus pneumoniae, Klebsiella pneumoniae, Haemophilus influenzae, and Neisseria meningitidis, known for their pathogenic potential and involvement in various clinically significant infections affecting human health.(36)
Capsule staining is a technique that aids in identifying encapsulated microorganisms and often contributes presumptively to diagnosis. The method involves treating the cell with specific stains that do not penetrate the capsule, which varies according to the technique used but generally contrasts the capsular structure against the bacterial cell.(7,37)
This approach was previously highlighted in the work of Lima and Teles,(38) who described the capsule’s composition, functionality, importance for classification, and correlation with capsular forms across different microorganisms. They also provided a historical and comparative discussion on various capsule staining techniques and their adaptations over time. In this article, the authors examine techniques developed by Rosenow(39) (1911) and Muir(40) (1915), which involved the use of gentian violet and eosin, as well as Smith,(38) who advocated for the use of these stains followed by methylene blue. With both theoretical and technical backing, Lima and Teles (1942) emphasized the difficulties of performing certain techniques at the time, associating these challenges with the low sensitivity of diagnostic methods.
The authors also referenced techniques described by Hiss(41) (1901) and Anthony(42) (1931) in their studies on methods for evaluating capsules produced by Klebsiella pneumoniae. Hiss(41) (1901) devised two distinct processes: the first proposed the use of gentian violet diluted to double its volume with distilled water, followed by rinsing with a 0.25% aqueous potassium carbonate solution; the second suggested staining with a heated aqueous solution of gentian violet or fuchsin, then washing with a copper sulfate solution. In Anthony’s technique(42) (1931), heat fixation was omitted, applying a 1% crystal violet solution as the initial stain for two minutes, followed by copper sulfate as a decolorizer, similar to Hiss’s method. Comparative studies by Lima and Teles(38) (1942) concluded that Anthony’s technique yielded better results, with larger and more visible capsules observed.
Currently, these older techniques are no longer in use. Among the main capsule detection methods described in the literature is negative staining, also known as the India ink method. This straightforward technique allows for the visualization of encapsulated bacteria without the need to stain them directly.(5,7,16) In bright-field microscopy, negative staining is typically performed using a black-pigmented fluid (acidic stain) like nigrosin or India ink, which cannot penetrate the cell, thus coloring the background and leaving the cells unstained. In this method, microorganisms are grown in a nutrient-rich medium, such as BHI (Brain Heart Infusion), to stimulate capsule production. The procedure involves placing 1 or 2 drops of this culture on a slide, followed by a drop of India ink (or nigrosin) adjacent to the culture. The slide is then covered with a coverslip and pressed between filter paper sheets to remove excess fluid, achieving a thin layer of stain and material. Observation under a microscope is carried out at magnifications of 4X, 10X, 40X, and 100X, respectively.(37) It is worth noting that, since live cells are used, biosafety measures must be strictly followed, including the use of PPE (personal protective equipment) when handling cultures and proper disposal of pipettes/tips and filter paper in a container designated for autoclaving.(5,7,16)
Another method described in the literature is a variation of the India ink method, using an additional stain, diluted fuchsin. In this approach, the initial steps are maintained, but a smear is prepared from the culture and India ink mixture, which, after drying, is stained with diluted fuchsin for two minutes. After this period, the slide is gently rinsed with water, dried, and examined under an optical microscope with an immersion objective.(5,7,16)
FLAGELLA STAINING
Flagella are locomotion structures formed by thin appendages made of protein (flagellin) and are found in motile bacteria. Each flagellum consists of thousands of polymerized monomers of this protein, arranged to form a single flagellum.(5,16)
Several challenges arise when attempting to visualize this type of organelle under optical microscopy, as bacterial flagella production is not continuous and depends on various factors, such as the culture medium, temperature, and growth stage.(5,16) Flagella are fragile and delicate structures that can easily detach from the cell body during abrupt pipetting and homogenization processes. Another critical factor causing detachment is their tendency to depolymerize readily, dissociating into flagellin monomers at temperatures above 60ºC, acidic pH (around pH 4.0), and in the presence of organic solvents, alkalis, and urea. Given these characteristics, specialized staining is required for flagella visualization, aiming to increase the flagellum diameter.
The principle of flagella staining relies on the use of tannic acid, which binds to the flagellum, increasing its diameter and thereby facilitating observation. Despite this enhancement, flagella still appear faint on the slide, making it challenging to capture clear images with light microscopy.(5,16)
The technique begins with a culture in a rich medium (typically BHI, as previously described for capsule staining) or on tryptic soy agar (with or without blood). A small aliquot of this culture is delicately transferred using a platinum loop into a tube containing approximately 3 mL of distilled water and inverted once, avoiding any abrupt movements to homogenize the suspension. Using a pipette, an aliquot is then carefully drawn by capillary action and allowed to flow naturally over the surface of a slide inclined at 45°, which is then left to air-dry. After this process, the slide should be covered with a staining mixture containing fuchsin and tannic acid, allowing it to act for 5 minutes until a greenish metallic sheen covers about half of the area. Subsequently, the stain is rinsed off with water before it dries on the slide, air-dried, and then observed under an optical microscope with an immersion objective (100X).(5,16)
IMPORTANCE OF LEARNING THESE METHODOLOGIES
As previously mentioned, despite significant advances in bacteriology in recent decades, including the development of molecular methods and automation that expedite clinical diagnostics and One Health research, the initial step of bacteriological analysis—microscopic examination of clinical material—remains a crucial stage.
As postgraduate instructors and experienced advisors, the authors have observed the difficulty students face in understanding the importance of microscopy, the principles of bacterial staining, and their relationship with the identification of agents, whether definitive or presumptive.
Therefore, we conceived this article as an opportunity to reinforce these topics, providing a historical perspective, state-of-the-art overview, practical applications, and the importance of understanding commonly used methods in research and laboratory routines. We believe this knowledge will add practical value for students in the health field, especially those directly involved in bacterial identification through various approaches.
MAIN LIMITATIONS AND QUALITY CONTROL IN STAINING TECHNIQUES
Quality control encompasses a set of measures, procedures, and tools applied during process execution to ensure proper performance. Therefore, the following recommendations should serve as mandatory requirements in daily routine activities:
1 – When performing any staining technique, control strains, such as those from the American Type Culture Collection (ATCC), should be used both to verify stain quality and to confirm proper methodology execution. Using cytomorphologically well-characterized strains is crucial for comparative sample recognition, enabling verification of similarities and/or differences in staining and morphological characteristics.
2 – Stains should be prepared according to recommended protocols and stored in amber bottles to protect them from light exposure, which can cause various alterations. Bottles should be well-sealed to prevent evaporation and stains should be routinely filtered to remove crystals that precipitate at the bottom. During staining procedures, avoid homogenizing the stain to prevent resuspension of any preformed crystals.
3 – Slides should be new, pre-washed, and degreased before use.
4 – Perform regular preventive maintenance and cleaning of microscopes and objectives after each session.
5 – The decolorization step is critical, as prolonged solvent exposure may result in complete stain removal, leading to unreliable results. The retention or removal of the primary stain depends on the physical and chemical properties of bacterial cell walls, including thickness, density, porosity, and integrity.
CONCLUSION
Despite all the current methodologies for bacterial detection and identification, basic staining techniques in microbiology still play a fundamental role in diagnosis, differentiation, and the study of microorganisms. The knowledge and proper application of these techniques are essential for microbiology professionals in different clinical and laboratory contexts. Each technique presents specific principles that allow for the visualization of distinct bacterial structures and has contributed to significant advances in medicine and biomedical research. Even in light of the undeniable importance of these techniques, the authors emphasize the need for more current studies aimed at developing methodologies that not only reduce the use of stains based on reagent costs but also seek to minimize the use of toxic substances, which will also contribute to more sustainable processes and environmental preservation.
REFERENCES
- Freitas V, Picoli S. A Coloração de Gram e as Variações na sua Execução. Novo Hamburgo. 2007; v.82 p124-128.
- Gram HCJ, Friedlaender C. Ueber die isolirte Färbung der Schizomyceten: in Schnitt-und Trockenpräparaten. Berlin: Theodor Fischer’s medicinischer Buchhandlung; 1884. (Fortschritte der Medicin; Bd. 2, 1884).
- Ziehl F. Zur Färbung des Tuberkelbacillus. Dtsch Med Wochenschr. 1882; 8:451-451.
- Kanaan S. Laboratório com interpretações clínicas. 2ª ed. Editora Atheneu; 2021.
- Nogueira JMR, Souza LF. Bacteriologia. In: Molinaro EM, Caputo LFG, Amendoeira MRR. Conceitos e Métodos para Formação de Profissionais em Laboratórios de Saúde. EPSJV – Fiocruz; 2009. p 221-397.
- Willey JM, Sherwood LM, Woolverton CJ. Prescott’s Microbiology. 8th. Ed McGraw-Hill; 2014.
- LeVeque RM, Martin N, Alst AJV, DiRita VJ. Microscopy and Staining: Gram, Capsule, and Endospore Staining. Em Cambridge, MA: JoVE Journal; Michigan State University; 2023.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 24 RD. Ed St. Louis, MO E-Book. Elsevier Health Sciences; 2021.ISBN: 9780323673204.
- Trento A. Colorações usadas em microbiologia [Internet]. [São José do Rio Preto – São Paulo]: Academia da Ciência e Tecnologia; 2018. Available at: http://www.ciencianews.com.br/arquivos/ACET/IMAGENS/Artigos_cientificos/3-Coloracao_microbiologia.pdf. Accessed: August 23, 2024.
- Teixeira AB, Cavalcante JCDV, Moreno ÍC, Soares IDA, Holanda FODA. Bacterial meningitis: an update. Rev Bras Análises Clínicas; 2018. 50 (4): 327-329. Available at: https://www.rbac.org.br/artigos/meningite-bacteriana-uma-atualizacao/. Accessed: September 02, 2024.
- Protocolo Clínico e Diretrizes Terapêuticas da Hanseníase. Brasília, DF: Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Ministério da saúde; 2022. 152p.
- Manual de Recomendações para o Diagnóstico Laboratorial de Tuberculose e Micobactérias não Tuberculosas de Interesse em Saúde Pública no Brasil. Brasília, DF: Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis Ministério da Saúde; 2022. 492 p.
- Hanseníase. Agência Fiocruz de Notícias. Lab de Hanseníase do Instituto Oswaldo Cruz. Fiocruz. Ministério da Saúde; 2024. Available at: https://portal.fiocruz.br/doença/hanseniase. Accessed: August 20, 2024.
- Morales PS. O tuberculosis, a coloração de Ziehl-Neelsen e a interpretação do BAAR [Internet]. Portal Afya. Available at: https://portal.afya.com.br/saude/o-mycobacterium-tuberculosis-e-a-classica-coloracao-de-ziehl-neelsen. Accessed: September 02, 2024.
- Rother ET. Revisão Sistemática x Revisão Narrativa. Editorial, Acta paul. enferm. 2007. 20 (2). Available at: https://www.scielo.br/j/ape/a/z7zZ4Z4GwYV6FR7S9FHTByr/ Accessed: September 02, 2024.
- Moreira JLB, Carvalho CBM, Frota CC. Visualização bacteriana e colorações. Ed. Imprensa Universitária – Universidade Federal do Ceará, CE; 2023. 68p
- Técnica de Coloração de Gram. Brasília, DF: Ministério da Saúde; 1997. 63p.
- Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov. 2019;3(2):275-90.
- Reine FU, Souza LABD, Menezes JLD, Gomes RFCC, Souza ACRD, Mota CA, et al. Método alternativo e sustentável para a realização de coloração bacteriana de Gram e Wirtz-Conklin: Relevância ambiental e econômica no ensino prático da microbiologia. Res Soc Dev. 22 de julho de 2021;10(9):e9510917585.
- Pinto AC, Ribeiro KTS. Guia prático de orientações básicas de microbiologia [Internet]. 1o Editora Itacaiúnas; 2022. Available at: https://editoraitacaiunas.com.br/produto/guia-pratico-de-orientacoes-basicas-de-microbiologia/. Accessed: September 20, 2024.
- Bush LM. Nocardiosis. MSD Manual, Charles E Schmidt Coll Med Florida Atlantic University. 2023. Available at: https://www.msdmanuals.com/home/infections/bacterial-infections-gram-positive-bacteria/nocardiosis. Accessed: September 23, 2024.
- Manual de recomendações para o controle da tuberculose no Brasil. Brasília, DF: Secretaria da Vigilância em Saúde. Departamento de Vigilância de Doenças Transmissíveis. Ministério da Saúde; 2019. 364p.
- Silva-Pereira TT, Soler-Camargo NC, Guimarães AMS. Diversification of gene content in the Mycobacterium tuberculosis complex is determined by phylogenetic and ecological signatures. Jun SR, organizador. Microbiol Spectr. 2024;12(2):e02289-23.
- Guia de Vigilância em Saúde. Brasília, DF: Secretaria da Vigilância em Saúde e Ambiente. Departamento de Ações Estratégicas e Epidemiologia e Vigilância em Saúde e Ambiente. Ministério da Saúde; 2024. 6ed. rev.v.3.
- Fontana A, Tribondeau P. Silver staining of spirochetes. Journal of Infectious Diseases. 1907.
- Morris SR. Sífilis.Manual MSD versão para profissionais de Saúde;2023. Available at: https://www.msdmanuals.com/pt-br/profissional/doenças-infecciosas/infecções-sexualmente-transmissíveis/sífilis. Accessed: September 23, 2024.
- Métodos de Coloração em Microbiologia [Internet]. Instituto Nacional de Medicina Laboratorial. 2023 [citado 16 de agosto de 2024]. Available at: https://inml.com.br/metodos-de-coloracao-em-microbiologia/. Accessed: September 23, 2024.
- Albert L, Laybourn A. A New staining method for the demonstration of volutin granules. Journal of Pathology and Bacteriology; 1934.
- Schaeffer AB, Fulton MD. A simplified method of staining endospores. 17 de fevereiro de 1933;77(1990):194. Available at: https://pubmed.ncbi.nlm.nih.gov/17741261/. Accessed: September 05, 2024.
- Wirtz R, Conklin R. A new method for ataining bacterial spores. Stain Technology; 1937.
- Hayama M, Oana K, Kozakai T, Umeda S, Fujimoto J, Ota H, et al. Proposal of a simplified technique for staining bacterial spores without applying heat – Successful modification of Moeller’s Method. Eur J Med Res. 2007.
- Martin BXB, Carraro DC, Souza DCR, Duarte EMPD, Ribeiro, SM, Gomes AB. Tipos de Botulismo: Uma revisão bibliográfica. Brazilian Journal Surgery and Clinical Research; 2019. Vol 26, N2, p 43-48.
- Silva ND, Junqueira VCA, Silveira NFDA. Manual de Métodos de Análise Microbiológica de Alimentos e Água [Internet]. 6o Blucher; 2021. Available at: https://www.blucher.com.br/livro/detalhes/manual-de-metodos-de-analise-microbiologica-de-alimentos-e-agua-1847. Accessed: September 20, 2024.
- Ministério da Saúde. Portaria GM/MS no 888, de 04 de maio de 2021. Altera o Anexo XX da Portaria de Consolidação GM/MS no 5, de 28 de setembro de 2017, para dispor sobre os procedimentos de controle e de vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Diário Of União. 2021;(85):127-127.
- Manual virtual de aulas práticas do DEMIP/ICBS/UFRGS [Internet]. Departamentos de Microbiologia, Imunologia e Parasitologia (Instituto de Ciências Básicas da Saúde) e Interdisciplinar (Campus Litoral Norte) da Universidade Federal do Rio Grande do Sul. 2024. Available at: https://www.ufrgs.br/aulaspraticasdemip/?page_id=845. Accessed: August 20, 2024.
- Cunha AMG. Análises clínicas. 2ª Vol. (Coleção Manuais de Farmácia, v.5). Salvador: Editora Sanar SA; 2021. 416 p.
- Barbosa FHF, Barbosa LPJL. Alternativas metodológicas em Microbiologia viabilizando atividades práticas. Revista de Biologia e Ciências de terra. Campina Grande. 2010; v10, n2, p 134-143.
- Lima JPDEC, Teles LQ. Demonstração de cápsulas bacterianas. Instituto Adolpho Lutz. 1942; 21p. Available at: http://www.ial.sp.gov.br/resources/insituto-adolfo-lutz/publicacoes/rial/40/rial-22_1942/b36.pdf. Accessed: September 17, 2024.
- Rosenow EC. A New Stain for Bacterial Capsules with Special Reference to Pneumococci. J Infect Dis. 1o de julho de 1911;9(1):1-8. Available at: https://academic.oup.com/jid/article-abstract/9/1/1/821027. Accessed: September 10, 2024.
- Muir R. Staining of bacterial capsules in films and sections. J Pathol Bacteriol. 1o de janeiro de 1915;20(2):257-9. Available at: https://onlinelibrary.wiley.com/doi/10.1002/path.1700200203. Accessed: September 20, 2024.
- Hiss PH. A Contribution to the physiological differentiation of Pneumococcus and Streptococcus, and to methods of staining capsules. J Exp Med. 1o de fevereiro de 1905;6(4-6):317-45. Available at: https://pubmed.ncbi.nlm.nih.gov/19866975/. Accessed: September 20, 2024.
- Anthony EE. A Note on Capsule Staining. Science. 1931;73(1890):319-20. Available at: https://www.science.org/doi/10.1126/science.73.1890.319. Accessed: September 20, 2024.
Correspondence
Joseli Maria da Rocha Nogueira
E-mail: [email protected]
